Benzene is a known human carcinogen. Long-term exposure to benzene has been linked to leukemia and other blood disorders. The FDA has drawn recent attention to Benzene contamination in consumer products such as sunscreens, hand sanitizers, and deodorants.
On July 27, 2022, the FDA announced that they are investigating the presence of benzene impurities in ~200 marketed drug products. These products include topicals including gels, creams, ointments and aerosol products such as sprays and foams. The method used for detection requires a Limited of Detection (LOD) ~100 ppb or lower.
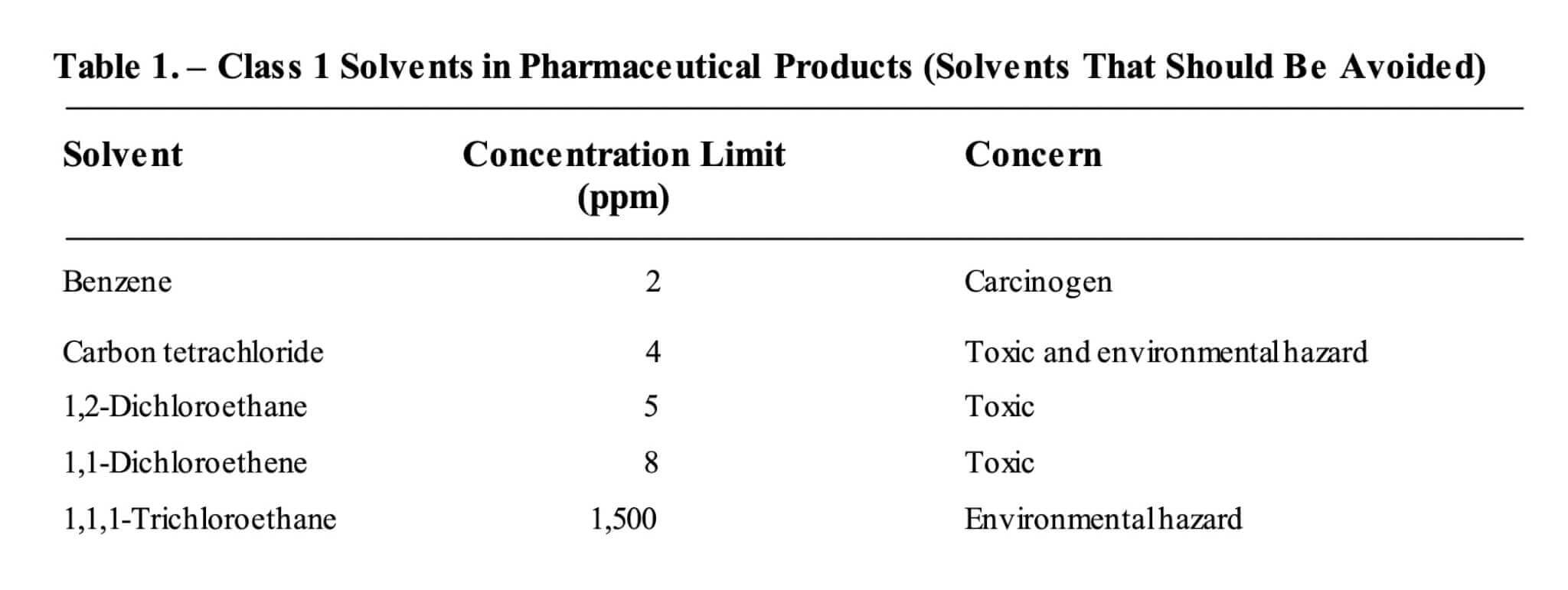
Recommendations outlined in the recent guidance ICH Q3C classify benzene as a Class 1 impurity, a solvent that should be avoided due to its carcinogenic and environmental hazard proprieties, as outlined below. The maximum allowable concentration is set at 2 ppm.
Analytical Procedures for Quantification:
- Quantitation of benzene using Headspace Gas Chromatography with Mass Spectrometry (HS-GC/MS)
- Analytical reference standard – benzene and benzene-d6 (internal standard).
- Instrument: Thermo Scientific TriPlus RSH Robotic Autosampler, TRACE 1300 Gas Chromatograph, ISQ 7000 Mass Spectrometer, and Chromeleon 7.3 software.
- Column: Restek Rtx-624, Length:30 m, ID: 0.32 mm, Film Thickness: 1.80 µm (Catalog #10970)
- Method: Headspace sampling with 60 °C 240 °C gradient.
Sample Preparation: 1) Dispense weighed product into appropriate vial, 2) Addition of DMSO solvent, 3) HS-GC/MS analysis. - Analysis: 7-point calibration with 3 external quality control samples to ensure assay robustness and accuracy. RT and Mass Spectral matching with certified reference standards.
- Recovery studies can be conducted upon request.
Causes of Benzene contamination:
According to the FDA, hydrocarbons or other ingredients manufactured with benzene or other hydrocarbons may indicate a higher likelihood of benzene contamination. Additionally, benzene contamination can result under certain conditions from an antifungal preservative called sodium benzoate.