Assignment of Complex NMR Spectra
Do you have a need for NMR services beyond basic 1-D proton spectra? Do you need expertise in analyzing and interpreting NMR data for discussions or presentations or publications? We can help by obtaining the specific 1-D and 2-D NMR experiments that will lead you to a high-level understanding of your compound. This will provide you with the information necessary to confidently make strategic decisions. We will be your partners in compound characterization.
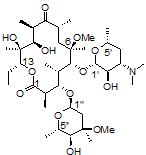
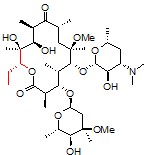
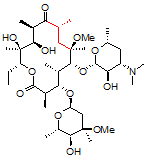
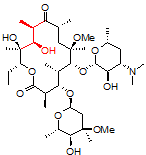
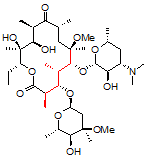
One challenging example of our services is shown below for the antibiotic clarithromycin. Clarithromycin is a semi-synthetic antibiotic which binds to the 50S ribosome, halting peptide synthesis in bacteria. A 14-membered macrolide, clarithromycin contains 38 carbons, 69 hydrogens, and 18 stereocenters, making analysis by NMR a fun challenge for most organic chemists and a perfect molecule to showcase our capabilities.
Obtaining 1H NMR and 13C NMR are straightforward on this compound, since the compound is very soluble, >10 mg/ml in CDCl3. In this example, only 128 scans were required to obtain a 13C, which is a demonstration of how sensitive and quick these newer spectrometers have become; however, when characterizing compounds for peer-reviewed documents (e.g., ACS journals), we recommend many more scans to increase the signal to noise ratio. For a complex molecule such as clarithromycin, pro-R and pro-S hydrogens on methylene groups are often separated by a large chemical shift difference and cause problems in the assignment. As a demonstration of our capabilities, we will be using 2-D NMR spectroscopy as an easier way to assign the peaks.
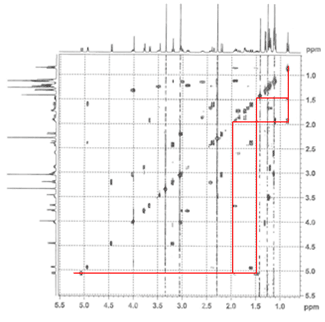
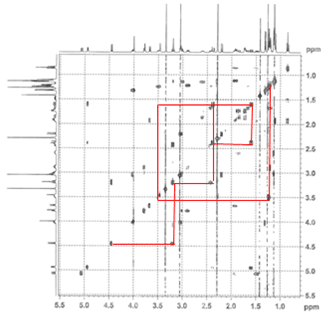
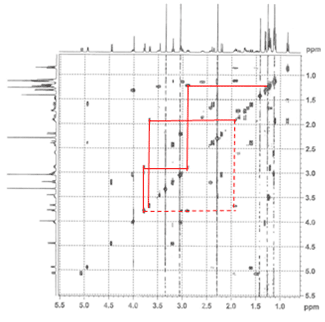
1. Correlation SpectroscopY (COSY) highlights 3-bond 1H-1H interactions and is useful for outlining different spin systems in a complex molecule. Clarithromycin has 7 different spin systems, C2-C5, C7-C8, C10-C11, C13, C1′-C6′, C1”-C2”, and C4”-C6”.
(d) The C7-C8 spin system is unique among the rest because there are no downfield-shifted protons.
The remaining three spin systems are similar in that all have the pattern CH3-CH-CH(OH), with the C2-C5 spin system having two such units. As a result, several of the peaks are overlapping, and assignment is difficult but possible.
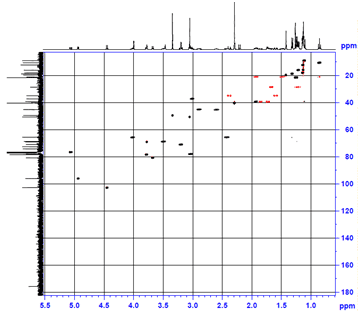
(e) The C4”-C6” spin system can be identified by the C5 proton, which is the most downshifted of all the remaining candidates. A multiplet at 4.02 ppm, it overlaps with an -OH signal, which we can determine from the HSQC (below).
(f-g) The C2-C5 spin system is differentiated from the C10-C11 spin system by the fact that it has an ester at one end (C1) rather than a ketone (C9). The two signals at 2.42 ppm and 2.89 ppm can be assigned to H10 and H2, respectively, and following the COSY cross-peaks helps us assign the final two spin systems.
The dashed lines show small cross-peaks which are only visible on a zoom-in.
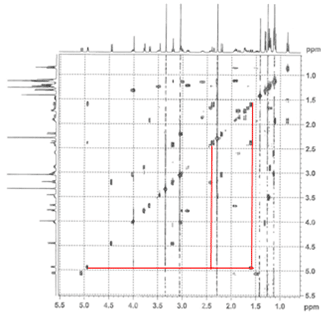
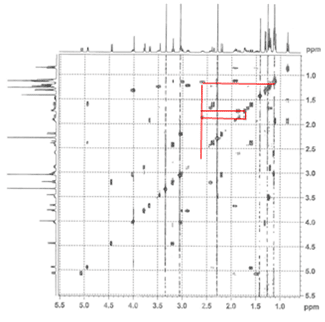
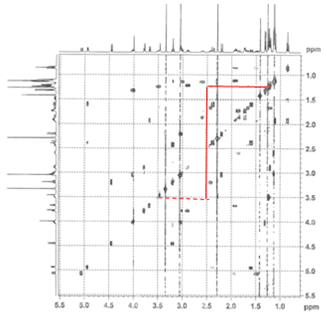
2. Heteronuclear Single Quantum Coherence (HSQC) spectroscopy highlights 1-bond1H-13C interactions (contrast to COSY, which highlights 3-bond 1H-1H interactions). Having assigned the 1H NMR spectrum, we can now assign the 13C NMR spectrum with a single experiment.
This particular HSQC is multiplicity-edited, which means that CH and CH3 groups are phased opposite of CH2 groups (much like a DEPT-135 experiment). This gives us additional information about the diastereotopic methylene protons, and, as referenced above, can be used in differentiating geminal from vicinal couplings in the COSY spectrum.
Organic chemists can often benefit from the use of 2-D NMR techniques. In particular, 2-D spectra can often help resolve bunched peaks (e.g., the methyl region from 1.0-1.2 ppm), as well as unambiguously assign closely related peaks (e.g., acetylation of the 2′-OH vs. 4”-OH).
If you’re interested in 2-D NMR and how it can help you with complex 1H NMR spectra, contact Emery Pharma.