Separation and Identification of alpha- and beta-glycopyranoside anomers
Many natural products consist of a protein, peptide, or a small molecule which has been glycosylated. These glycosylations can happen for a variety of reasons, including cell-cell recognition of glycosylated proteins (e.g., blood type is determined by glycosylation patterns), marking a protein for export (e.g., in the endoplasmic reticulum), or simply to increase potency (e.g., macrolide antibiotics, whose C3-deglycosylated analogs are inactive).
Glycotransferases take an activated sugar, and append it onto the core structure. The resulting linkage can be alpha (cis stereochemistry between the C1 substituent and C6-C5 bond), or beta (trans stereochemistry between the C1 substituent and C6-C5 bond), depending on the nature of the enzyme. Chemical synthesis of sugars and glycosylated products may generate a mixture of alpha and beta products through an sp2-hybridized oxonium intermediate.
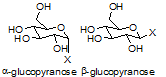
The analytical challenge is to separate (when necessary) and identify mixtures of alpha- and beta-glycosides. In this study, we analyze a mixture of 4-methylumbelliferyl alpha- and beta-glucopyranosides by LCMS and NMR.
Separation of the anomers is not trivial, but is straightforward method development work. Figure 1 shows a 1:1 mixture of the alpha- and beta- anomers run on our standard 50 mm column. The two compounds co-elute, and are inseparable by this method (5-95% acetonitrile).
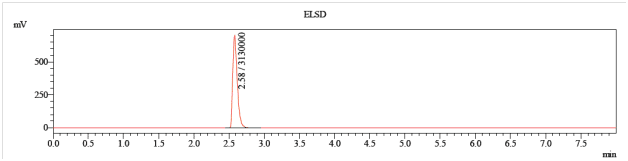
Figure 1: A mixture of alpha- and beta- anomers on a standard run (50 mm C18, 5-95% acetonitrile in water)
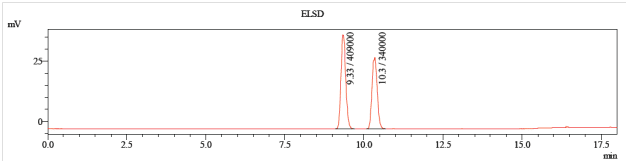
Figure 2: The same 1:1 mixture after method development (150 mm C18, 5-10% acetonitrile in water). Mass spectrometry (not shown) confirms that both compounds have the same mass-to-charge ratio and are isomers.
These anomers are separable by preparatory HPLC with this gradient (data not shown).
After separation, determination of which anomer is the alpha-glucoside and which is the beta-glucoside can be done in several ways. The most reliable method is JCH analysis of the C-1 and H-1 atoms. An equatorial arrangement typically leads to a higher JCH (~170 Hz) than an axial arrangement (~160 Hz).
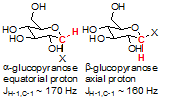
Identification of the H-1 and C-1 signals has been covered in other posts, and details of those procedures can be found in the linked pages. Briefly, H-1 was identified with a COSY spectrum by "walking" down the pyranoside backbone. The C-1 was then readily identified from a 1H-13C HSQC spectrum showing 1-bond correlation of the H-1 with C-1. In this case, the C-1 signal of the earlier-eluting anomer was at δ 100.47 ppm, while the C-1 signal of the later-eluting anomer was at δ 98.25 ppm.
As a side note, based on the chemical shifts alone, we can suspect the earlier eluting anomer to be the β-anomer, as the β-anomers typically have higher chemical shifts than their corresponding α-anomers. However, these rules of thumb are described for natural polysaccharides in which the anomeric substituent is another sugar. In the case of glycosylated small molecules, the chemical shifts are different enough (98-100 ppm in this case vs. 70-76 ppm for a typical polysaccharide) to be wary of comparisons to the natural compounds. To be rigorous, we want an absolute method of determination, not just a relative method.
The JCH couplings can be derived by comparison of the standard 13C NMR spectrum with a decoupled 13C NMR spectrum. Standard 13C NMR spectroscopy uses proton-decoupling (irradiation of the proton channel), accomplishing both (a) increased signal-to-noise by nuclear Overhauser effect (NOE), and (b) increased signal-to-noise by collapsing the coupled signal into one decoupled signal. A completely-decoupled 13C NMR spectrum can be acquired, but suffers from extremely low signal-to-noise. Because the NOE primarily occurs during the recycle delay (between the previous data acquisition and the next pulse) and the decoupling primarily occurs during the acquisition, gated decoupling gives a significant boost to signal-to-noise while preserving the C-H couplings.
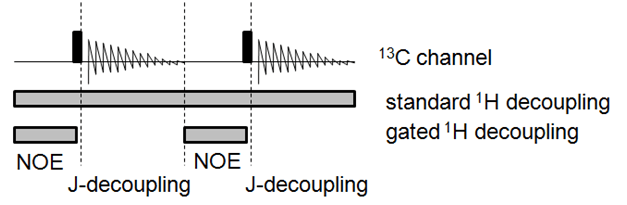
Analysis of the coupled (gated-decoupled) 13C NMR spectrum is straightforward. The chemical shift is given in ppm, but the peaks are labeled in Hz for clarity in calculating the coupling constants.
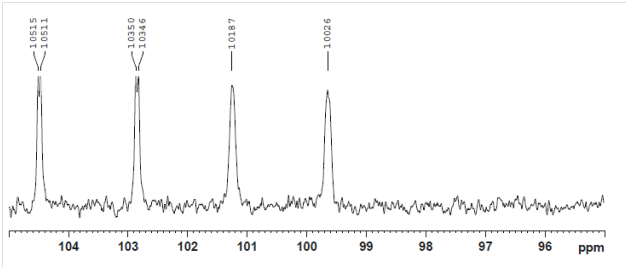
Early-eluting anomer: JCH = 161 Hz => β-glucoside
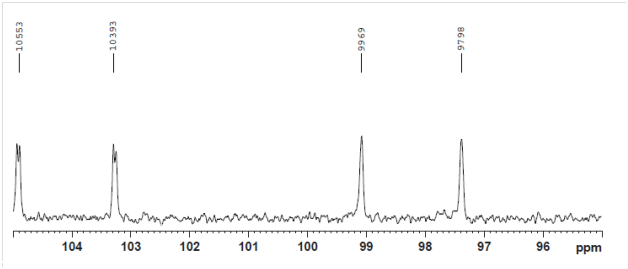
Late-eluting anomer: JCH = 171 Hz => α-glucoside
Using a combination of LCMS and NMR, we have separated and identified two anomers of 4-methylumbelliferyl alpha- and beta-glucopyranosides. The two anomers were separated on a C18 column, and comparison of the gated-decoupled 13C NMR spectra showed that the early eluting isomer was the beta-glucoside and the late-eluting isomer was the alpha-glucoside.
If you’d like to know more about gated-decoupled 13C NMR spectra, glycosidic bond analysis, or LCMS or NMR analysis in general, contact Emery Pharma. We’d love to help you with your challenging problems.
