Emery Pharma Ranitidine: FDA Citizen Petition
Alameda, CA – On January 2, 2020, Emery Pharma filed a Citizen Petition with the FDA regarding the drug Ranitidine, which appear to produce unacceptably high levels of a cancer-causing chemical when exposed to heat. Ranitidine is a common heartburn medication and is sold under various brand names, including Zantac®.
In an ongoing study, Emery Pharma found that Ranitidine is a “Time- and temperature-sensitive pharmaceutical product (TTSPP)”, which develops a known carcinogen called N-Nitrosodimethylamine (NDMA) when exposed to heat. Exposure to high temperatures is a common occurrence during transportation and storage, and is of specific concern to ranitidine as there currently are no requirements for the drug to be shipped in temperature-controlled conditions and/or stored under refrigeration.
“Even limited exposure of Ranitidine to high heat, such as in a hot car or a delivery truck, could cause problems” – Dr. Ron Najafi, CEO, Emery Pharma.
In the citizen petition, Emery Pharma is urging the FDA to suspend sales of all products containing ranitidine, to recall those that are already on the market, and to require additional stability testing before any future sale. Further, Emery Pharma urges that the drug be shipped in temperature-controlled vehicles and carry warnings that potential cancer-causing byproducts may be produced if it is exposed to heat.
For all press inquires, please contact:
Neeku Mahdavian
neeku@emerypharma.com
Tel: (510) 899-8825
The entire Citizen Petition is shown below:
January 2, 2020
Division of Dockets Management
Food and Drug Administration
5630 Fishers Lane, Room 1061
Rockville, MD 20852
Re: Emery Pharma Citizen Petition
To Whom It May Concern:
The undersigned, on behalf of Najafi Pharma, Inc. dba Emery Pharma (collectively, “Emery Pharma” or “Petitioner”), submits this Citizen Petition (“Petition”) pursuant to Sections 301(21 U.S.C. § 331), 502 (21 U.S.C. § 352), 505 (21 U.S.C. § 355), 702 (21 U.S.C. § 372), 704 (21 U.S.C. § 374), and 705 (21 U.S.C. § 375) of the Federal Food, Drug and Cosmetic Act (the “FDCA”), in accordance with 21 C.F.R. 10.20 and 10.30, to request the Commissioner of Food and Drugs (“Commissioner”) to issue a regulation, revise industry guidance, and request a recall and suspend sales of ranitidine from the US market and take such other actions set forth below.
A. Action Requested
The drug ranitidine, commonly sold under the brand Zantac®, is an antihistamine, specifically an H₂ histamine receptor blocker (“H2 blocker”) that is extensively used for heartburn and other stomach acid-related diseases.¹ Due to its established safety profile and efficacy for treating these diseases, ranitidine is on the World Health Organization’s Model List of Essential Medicines.² However, Emery Pharma has recently conducted a preliminary stability study that indicates that the ranitidine molecule may not be heat stable, and under elevated temperatures, generates significant amounts of N-Nitrosodimethyl-amine (“NDMA”), a probable human carcinogen.
On September 9, 2019, an FDA citizen petition was posted by a pharmacy, reporting extremely high levels of N-Nitrosodimethylamine (“NDMA”), irrespective of the manufacturer, manufacturing lot, dosage, and formulation of ranitidine (“September 9 Citizen Petition”).³ As the Food and Drug Administration (“FDA”) has established, the daily intake limit for NDMA should be less than 96 ng, whereas the September 9 Citizen Petition noted NDMA in excess of 3,000,000 ng per tablet when analyzing ranitidine products, which the company submitting the document hypothesized may be due to inherent instability of the molecule at high temperatures. Subsequently, FDA opined on October 2, 2019 that the headspace GC-MS (“HS-GC-MS”) methodology used in the September 9 Citizen Petition was not suitable for ranitidine analysis, as the method requires incubation of the drug at elevated temperatures (130 ⁰C or higher).⁴ Instead, the agency published a complementary LC-HRMS method for detection and quantification of NDMA in ranitidine drug substance and products. Using this method, FDA detected much lower levels of NDMA in ranitidine, yet still found unacceptable levels of NDMA in some lots of the drug.
Emery Pharma is an FDA registered, GLP/cGMP compliant laboratory. We proceeded with a detailed investigation of the stability of ranitidine and potential for generation of NDMA in vitro. Specifically, we were interested in the fact that HS-GC-MS revealed that ranitidine may be heat unstable. More importantly, we wanted to understand, if not as an analytical artifact, how unacceptable levels of NDMA were detected in certain lots of ranitidine by the FDA.
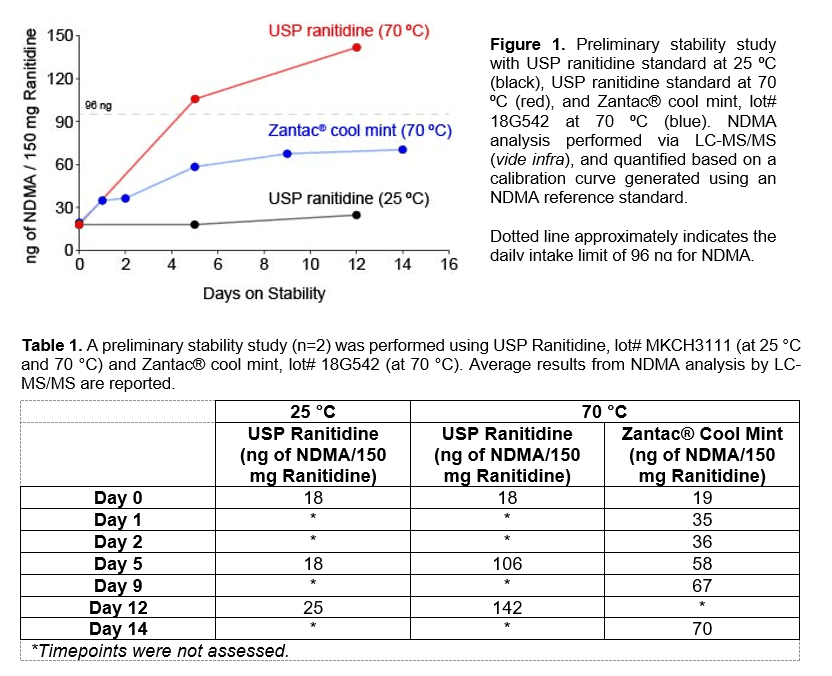
Emery Pharma’s preliminary analysis revealed that while seemingly stable at room temperature, the ranitidine molecule is heat-labile and under elevated temperatures (e.g., 70 ⁰C) progressively accumulates NDMA (Figure 1 and Table 1).
This was concerning, since significantly elevated temperatures can occur within closed vehicles during transportation and during storage of the drug, because there is no requirement for ranitidine to be cold-chained, i.e., shipped in temperature-controlled conditions and stored under refrigeration. The drug is very likely exposed to elevated temperatures during shipment and commercial storage as well as post-purchase storage by consumers. Of particular concern is shipment through or storage in the southern states of continental US, as well as countries such as in the Middle East, Southeast Asia, and the equatorial region that experience significantly elevated temperatures throughout the year.
FDA’s recent directive, dated December 4, 2019⁵, states:
“Today, we are announcing that we have asked manufacturers of ranitidine and nizatidine products to expand their testing for NDMA to include all lots of the medication before making them available to consumers. If testing shows NDMA above the acceptable daily intake limit (96 nanograms per day or 0.32 parts per million for ranitidine), the manufacturer must inform the agency and should not release the lot for consumer use.”
Our preliminary data indicate that NDMA accumulates in ranitidine-containing drug products on exposure to elevated temperatures, which would be routinely reached during shipment and during storage. More importantly, these conditions occur post-lot release by the manufacturer. Hence, while NDMA levels in ranitidine may be acceptable at the source, they may not be so when the drug is purchased and subsequently at the time of consumption by the consumer.
A potential solution to this issue would be testing each batch of ranitidine products for NDMA at the site of sale, as opposed to testing only by the manufacturer at the production site. However, Emery Pharma recognizes that this could be impractical for many retailers. To avoid exposure to elevated temperatures, ranitidine drug substances should be cold-chained, i.e., shipped in temperature-controlled trucks and stored under refrigeration. The products should also be labeled with clear warnings regarding storage conditions. In addition, the products could also be required to carry a temperature indicator label that would inform the consumer of excessive exposure to heat.
This Petition requests that the Commissioner take the following actions:
- Request a recall and suspend sale of all lots of all products containing ranitidine. Given ranitidine’s propensity to deteriorate at elevated temperatures to the probable carcinogen NDMA, the drug is misbranded under Section 502 of the FDCA (21 U.S.C. § 352(h));
- Conduct examinations and investigations under Section 702 (a) of the FDCA (21 U.S.C. § 372(a)) regarding ranitidine products, specifically stability assessment and the manufacturer submissions made for FDA approval under 704(a) of the FDCA (21 U.S.C. § 374(a));
- Provide information to the public regarding the high temperature instability of ranitidine products under Section 705(b) of the FDCA (21 U.S.C. § 375(b));
- In addition to the instructions for disposal and/or return in the recall notices, issue additional guidance to the public for the safe disposal of ranitidine, given the recognized potential that the drug may degrade to form the probable carcinogen NDMA in municipal wastewater treatment plants and impact the public water supply as was cited in the September 9 Citizen Petition;
- Issue a directive to the manufacturers of ranitidine products to conduct a thorough stability assessment of the compound towards formation of NDMA, both in drug substance and drug product forms;
- Issue a directive to the manufacturers and distributors to ship ranitidine products in temperature-controlled vehicles;
- Issue a directive to the manufacturers to clearly label ranitidine products with a warning, such as: “by-products that are probable carcinogens can be generated if exposed to heat;” and
- In the interest of public safety, require that ranitidine-containing products be moved behind the counter and dispensed by “prescription only” and ideally tested for NDMA or otherwise assessed for heat exposure (as with a temperature-indicator label) at the dispensing pharmacy and not just at the manufacturing site.
Background on Petitioner
Emery Pharma is an FDA-registered and inspected, GLP/cGMP-compliant, and DEA-licensed laboratory. We have significant expertise in analytical chemistry (UPLC, LC-MS, NMR), medicinal chemistry, structure elucidation, and formulations. We are also highly experienced in method development, sample preparation, and validation for the analysis of drugs in manufacturing lots, blood, plasma, urine, and tissues.
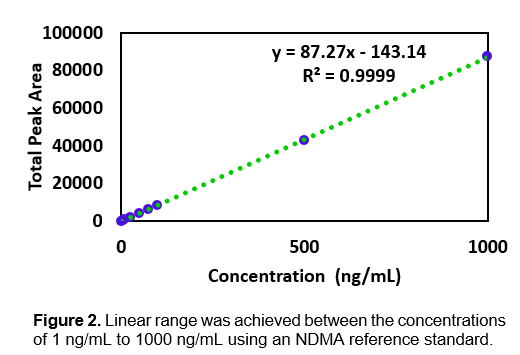
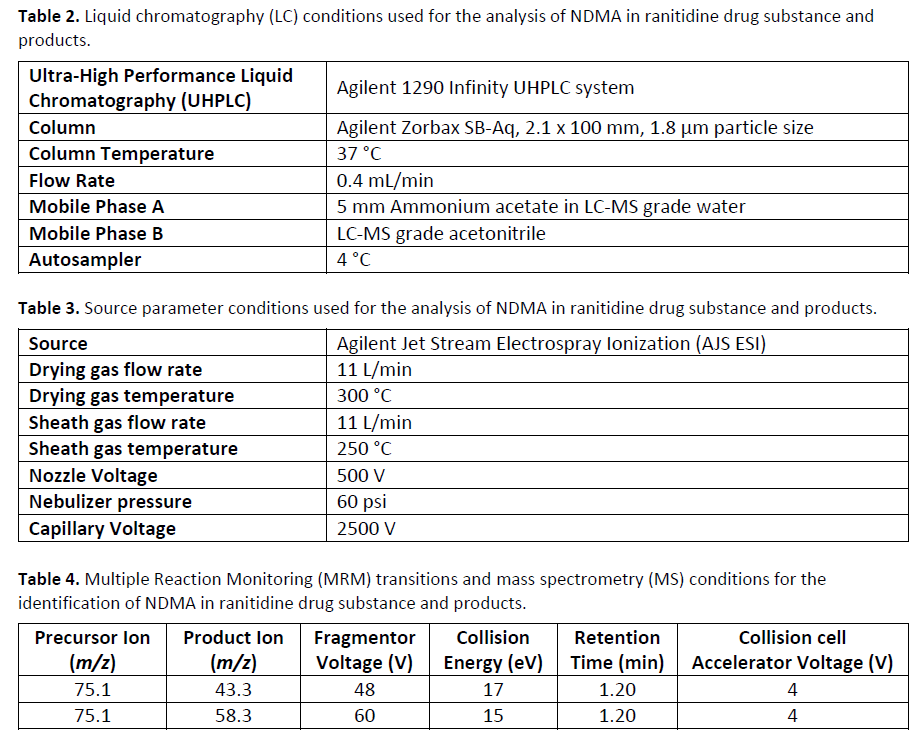
Emery Pharma was contracted to test for NDMA in Valsartan.⁶ The high temperatures used in the traditional HS-GC-MS protocol for studying NDMA in drug products, as indicated by FDA in its press release on October 2, 2019, has the potential for artefactual accumulation of NDMA in ranitidine during HS-GC-MS analysis. Paralleling FDA’s efforts in the matter, Emery Pharma pursued the development and validation of an Ultrahigh Performance Liquid Chromatography (“UPLC”) – Multiple Reaction Monitoring (“MRM”, a type of MS/MS) method for NDMA that was designed to maintain ranitidine under refrigerated conditions (as opposed to heating at 130 ⁰C as with HS-GC-MS) during analysis. See the methodological details provided in Figure 2 and Tables 2, 3, and 4.
Emery Pharma next embarked on a stability assessment for ranitidine under elevated temperature conditions, specifically looking into the potential for ranitidine to generate NDMA at elevated temperatures. This study is still ongoing, and being prepared for a peer-reviewed manuscript. However, in light of FDA’s recent directive from December 4, 2019, Emery Pharma is using this Petition to bring certain preliminary data to the agency’s attention⁷:
- As noted in Figure 1, ranitidine (as drug substance and product) shows a substantial and steady accumulation of NDMA when incubated at 70 ⁰C, whereas no significant increase in NDMA was observed at 25 ⁰C.
- NDMA accumulation in ranitidine was observed in samples of ranitidine USP reference material and Zantac® pills tested.
- Stability upon exposure to high temperatures (e.g. 70 ⁰C) is highly relevant for drug products, including ranitidine pills, as such temperatures are likely routinely achieved during product shipment and storage (both commercial, as well as in-vehicle and other storage by end-users). This is particularly of concern at warmer regions of the world, e.g. southern states of continental US, as well as countries such as in the Middle East, Southeast Asia, and the equatorial region.
- The propensity for ranitidine to generate NDMA at 70 ⁰C suggests that such accumulation may be possible at lower temperatures, albeit at a slower rate, and there should be strict requirements in product labeling to indicate appropriate storage conditions.
These conclusions derive from a preliminary and ongoing stability assessment. Emery Pharma expects to complete the study over the next few weeks and is preparing the data for a peer-reviewed publication. In light of FDA’s recent directive of December 4, 2019, Emery Pharma seeks to utilize this Petition to bring these stability concerns directly to the attention of the Commissioner and the FDA, and request that they take expeditious action.
B. Statement of Grounds
The World Health Organization (“WHO”) and the International Agency for Research on Cancer (“IARC”) have classified NDMA as a Group 2A compound thereby defining it as “probably carcinogenic to humans.”⁸ Furthermore, an epidemiological study has implicated ranitidine’s drug class as being correlated to cancer.⁹
The FDA currently recognizes the danger of such compounds and, as a result, has set strict daily acceptable intake limits on NDMA in pharmaceuticals of 96 nanograms.¹⁰ There have been extensive recalls of angiotensin receptor blocker (“ARB”) drugs, such as Valsartan, due to the detection of NDMA above these limits.¹¹ It is important to note that the recommended dosage for ranitidine, depending on the stomach disease in question, may be 150 mg orally 2 times (common) or even 4 times a day, with a maximum dose up to 6 g of ranitidine/day.¹² Hence, with the daily intake limit of NDMA being 96 ng/day, the acceptable limit of NDMA should be at most 48 ng / 150 mg ranitidine tablet, or perhaps lower.
Emery Pharma’s preliminary stability assessment on ranitidine suggests that the compound is prone to degradation to generate the potential carcinogen, NDMA, at temperatures that are likely routinely achieved during product shipment and commercial storage as well as storage by consumers, particularly in the warmer regions of the world. Given this observation, depending on shipping and storage conditions, ranitidine pills may accumulate NDMA in excess of FDA’s acceptable daily intake limit. Petitioner urges the Commissioner and the FDA to update its December 4, 2019 directive on testing ranitidine products at the source and send expeditious recall requests for all ranitidine products until the stability of the product, storage and shipping conditions have been properly evaluated. We request this to protect the American public, as well as the global consumer base from further exposure to the potentially carcinogenic properties of ranitidine, which is not labeled for such risk or appropriate storage conditions.
C. Environmental Impact
Petitioner claims a categorical exclusion under 21 C.F.R. § 25.30, and believes that this Petition qualifies for a categorical exclusion from the requirement to submit an environmental assessment or environmental impact statement.
D. Economic Impact
Pursuant to 21 C.F.R. § 10.30(b), economic impact information will be submitted by the Petitioner only upon request of the Commissioner following review of this Petition.
E. Certification
The undersigned certifies that, to the best knowledge and belief of the undersigned, this Petition includes all the information and views on which the petition relies, and that it includes representative data and information known to the petitioner which are unfavorable to the petition.
Respectfully submitted,
Ramin (Ron) Najafi, Ph.D.
President and CEO
Emery Pharma
1000 Atlantic Ave., ste 110
Alameda, CA 94501
Phone: (415) 747-2087
E-mail: ron@emerypharma.com
Sources:
- “Ranitidine Hydrochloride Monograph”. The American Society of Health-System Pharmacists. Archived from the original on 9 September 2017 (https://www.drugs.com/monograph/ranitidine-hydrochloride.html).
- https://www.who.int/medicines/publications/essentialmedicines/en/
- https://www.regulations.gov/document?D=FDA-2019-P-4281-0001.
- https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-zantac-ranitidine.
- https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-ndma-zantac-ranitidine.
- https://www.regulations.gov/document?D=FDA-2019-P-2869-0001.
- Emery Pharma is conducting this ongoing stability assessment of ranitidine and any associated UPLC-MS/MS method development work independently, and these efforts are not supported financially by Valisure LLC (the company that submitted the prior citizen petitions referenced above) or any of Emery Pharma’s clients.
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans (1987). International Agency for Research on Cancer and World Health Organization. Vol. 17, Supp. 7 (https://monographs.iarc.fr/list-of-classifications-volumes/) and Preamble to IARC Monographs on the Identification of Carcinogenic Hazards to Humans.(2019). World Health Organization. (https://monographs.iarc.fr/wp-content/uploads/2019/01/Preamble-2019.pdf)
- Michaud, D.S., Mysliwiec, P.A., Aldoori, W., Willett, W.C., Giovannucci, E. (2004). Peptic ulcer disease and the risk of bladder cancer in a prospective study of male health professionals. Cancer Epidemiol Biomarkers Prev. Vol. 13, 2, p. 250-254. (https://www.ncbi.nlm.nih.gov/pubmed/14973090).
- FDA updates table of interim limits for nitrosamine impurities in ARBs (February 28, 2019). US Food and Drug Administration. (https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan)
- Search List of Recalled Angiotensin II Receptor Blockers (ARBs) Including Valsartan, Losartan and Irbesartan. (current as of 06/28/2019). US Food and Drug Administration. (https://www.fda.gov/drugs/drug-safety-and-availability/search-list-recalled-angiotensin-ii-receptor-blockers-arbs-including-valsartan-losartan-and)
- https://www.drugs.com/dosage/ranitidine.html.