What is Peptide Mapping?
Peptide mapping is a powerful analytical technique used to unravel the intricacies of proteins. It involves digesting a protein using enzymes like trypsin, Lys-C, Glu-C, or chymotrypsin. This enzymatic digestion breaks the protein into smaller fragments known as peptides. These peptides are then separated by HPLC and analyzed via mass spectrometry, providing critical insights into the protein's primary structure and composition.
Why Peptide Mapping?
Peptide mapping plays a pivotal role in protein characterization and provides detailed information about the primary amino acid structure of proteins. Further, peptide mapping is indispensable for confirming identity, providing primary structural insights, and ensuring quality assurance and quality control (QA/QC) of biopharmaceutical such as monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs). More specifically, peptide mapping can be used for:
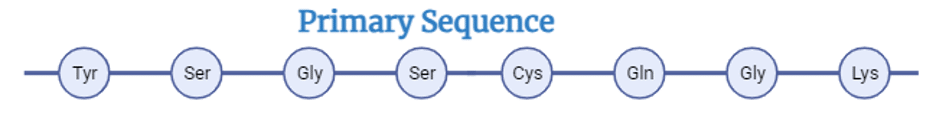
- Confirming the Primary Sequence:Peptide mapping can confirm the primary sequence of the protein and identify any errors or misincorporations in the amino acid sequence, which can affect the protein function and stability. These errors can occur due to mutations, transcription errors, or translation errors, and they can have significant implications for the quality of biopharmaceutical products.
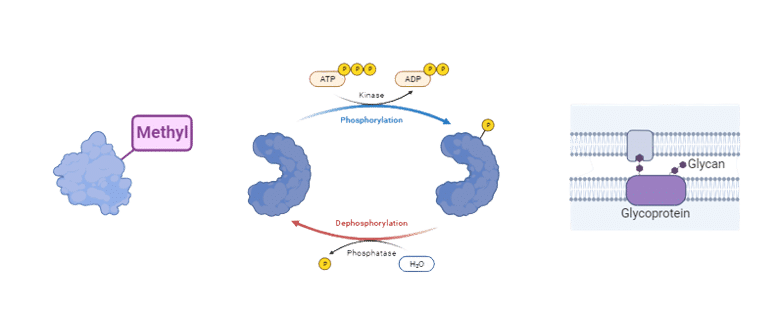
- Identifying Post Translational Modifications:Peptide mapping can identify post translational modifications (PTMs) of the protein, such as phosphorylation, deamidation, oxidation, ubiquitination, nitrosylation, methylation, acetylation, lipidation and glycosylation. PTMs are changes in the protein structure that regulate its function and have various implications for research and drug development. For example, PTMs can affect the protein activity, interactions, localization, stability, and signaling.
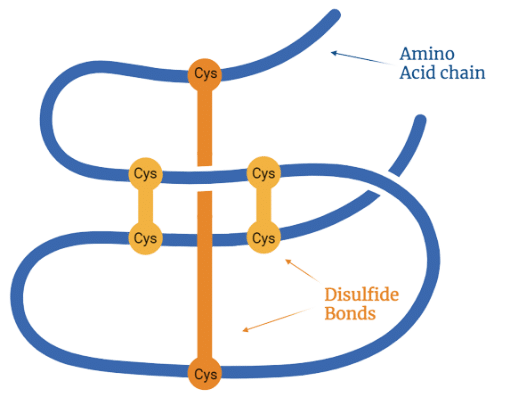
- Mapping Disulfide Bonds:Peptide mapping can map the location of disulfide bonds in the protein, which are covalent bonds that stabilize the protein structure and influence its folding and activity. Disulfide bonds are formed between cysteine residues in the protein sequence, and they can be intra-chain or inter-chain. Peptide mapping is used to determine the number and position of disulfide bonds by comparing the mass spectra of reduced and non-reduced peptides.
- Detecting Impurities:Peptide mapping can detect impurities or contaminants in the protein sample, even at very low concentrations. Impurities can arise from various sources, such as degradation products, host cell proteins, endotoxins, or residual solvents. Peptide mapping can identify these impurities by comparing the mass spectra of the sample with a reference standard or a database. Impurities can affect the safety and efficacy of biopharmaceutical products, so it is important to monitor them during development and manufacturing.
LC-MS Advantages
LC-MS-based peptide mapping offers unparalleled specificity and detailed structural information.
Specifically:
- High Sensitivity: LC-MS-based peptide mapping excels in detecting even trace amounts of peptides and proteins. This high sensitivity is invaluable for the analysis of samples with limited availability or when identifying impurities at low concentrations.
- Accurate Mass Measurement: Accurate mass determination is crucial for confident identification of peptides and post-translational modifications. Advanced instruments, such as high-resolution mass spectrometers, provide precise mass measurements, enhancing the reliability of results.
- Relative Quantitative Analysis: The technique facilitates relative quantitative analysis, enabling comparisons of peptide or protein abundances across different samples. This is essential for understanding changes in expression levels or the relative abundance of post-translational modifications in various conditions.
- Robust Across Different Matrices: LC-MS-based peptide mapping is a versatile and robust method applicable across diverse sample matrices. This adaptability is particularly beneficial in proteomics, where samples and research questions can vary widely.
- Detection of Diverse PTMs: LC-MS-based peptide mapping can identify and quantify a wide range of post-translational modifications (PTMs) not easily detectable by other methods, including phosphorylation, ubiquitination, methylation, acetylation, and sulfonation. This capability aids in the comprehensive characterization of proteins.
Our Expertise
At Emery Pharma, we leverage our extensive experience in LC-MS-based peptide mapping studies. We use state-of-the-art technology, including the Orbitrap Exploris™ 240 Mass Spectrometer, to deliver precise and reliable results. Whether you need detailed characterization of your protein, identification and quantification of post translational modifications, or detection of impurities, we can provide you with the best solution for your needs. We offer customized services, comprehensive reports, and data analysis/interpretation. Contact us today to find out how we can help you with your peptide mapping project.
Example :
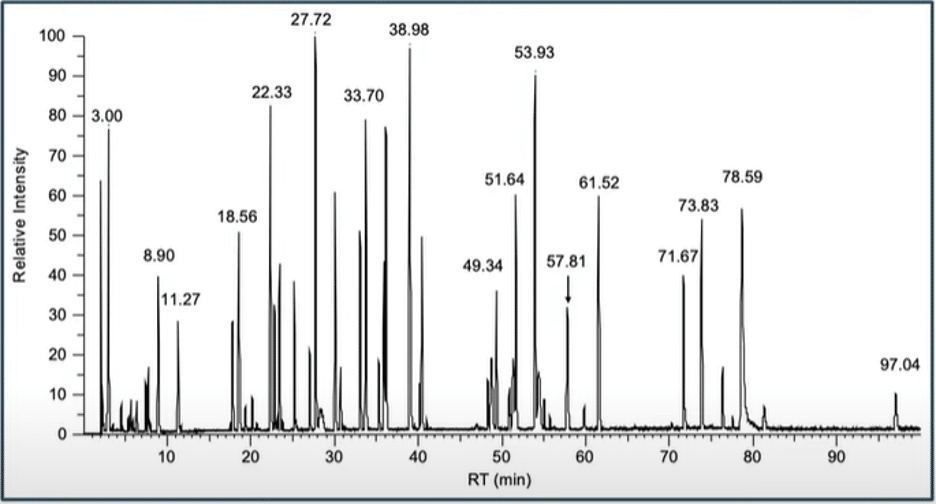
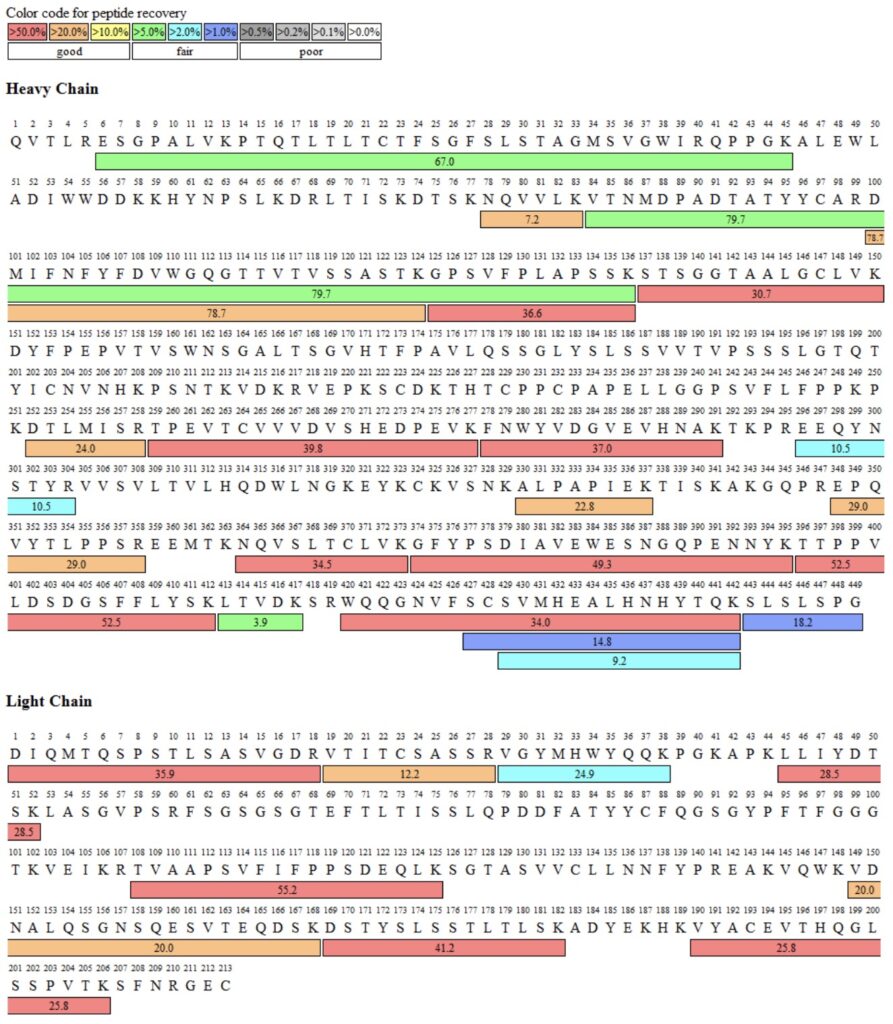
The following spectra were obtained from a peptide mapping project on the NIST Monoclonal Antibody Reference Material 8671. All results were obtained here at Emery Pharma.
LC-Chromatogram after tryptic digest:
Sequence Coverage Map after tryptic digest: