Understanding Antimicrobial/Preservative Effectiveness in Everyday Products

Toothpaste, lotions, cosmetics, eye drops, and medications are just some of the products that we all use on a daily basis. Unfortunately, there are two ways that these products can get contaminated: during the manufacturing process or during usage. During usage, microbial contamination occurs when the product is constantly opened and closed. These products are potentially exposed to many types of microbes, from airborne fungal spores to the bacteria on your fingertips. These microbes need few nutrients to grow and thrive.
To prevent microbial growth and preserve product stability, preservatives are added to these products to prevent contamination and ensure product safety and longevity. Preservatives are also commonly found in many food and beverage products. Some of the most commonly used preservatives include benzalkonium chloride, chlorhexidine, sodium benzoate, thimerosal, sorbic acid, citric acid, and benzoic acid. These ingredients create an antimicrobial environment to inhibit the growth of harmful organisms.
To evaluate the effectiveness of these preservatives, a standardized microbial challenge test known as United States Pharmacopeia-51 (USP <51>), or the Antimicrobial Effectiveness Test (AET), is used. The AET determines whether common microorganisms can survive and proliferate in a preserved product over time. The test panel includes: Aspergillus brasiliensis ATCC 16404 (black mold), Candida albicans ATCC 10231 (yeast), Escherichia coli ATCC 8739 (a gut bacterium), Pseudomonas aeruginosa ATCC 9027 (opportunistic pathogen), and Staphylococcus aureus ATCC 6538 (common cause of skin infections).
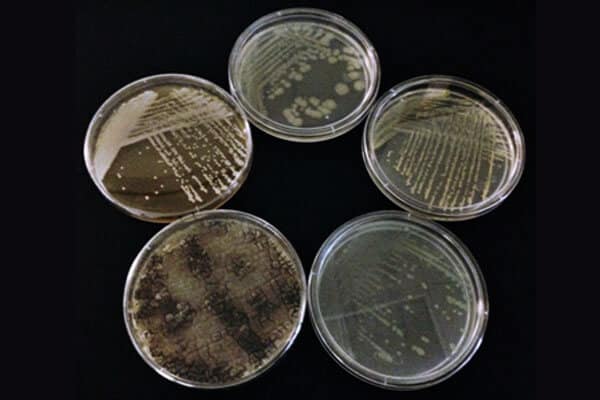
Figure 1: (clockwise from the top) Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6538, Pseudomonas aeruginosa ATCC 9027, Aspergillus brasiliensis ATCC 16404, and Candida albicans ATCC 10231 grown in the Emery Pharma microbiology lab.
AET quantitatively assesses preservative effectiveness against microbial growth. Emery Pharma conducts AET in accordance with USP <51>, European Pharmacopeia 5.1.3, and Japanese Pharmacopeia 19. A harmonized protocol [1] integrates all pharmacopeial standards. The inoculum must not exceed 1% of the test material. Bacterial tests are incubated at 35°C, fungi at 25°C. Time points include days 7, 14, and 28 for USP and JP, with additional 6 and 24-hour points required by EP. Samples are plated on appropriate agar media such as Soybean-Casein Digest Agar and Sabouraud Dextrose Agar to assess viability.
To pass the AET, results must show that A. brasiliensis and C. albicans do not exceed initial counts, while bacterial counts must show a 1–3 log reduction, depending on the product type as detailed in USP <51> Tables 1 and 2.
Table 1: Compendial Product Categories
|
Category |
Product Description |
|
1 |
[Injectables], other parenterals including emulsions, otic products, sterile nasal products, and ophthalmic products made with aqueous bases or vehicles. |
|
2 |
Topically used products made with aqueous bases or vehicles, nonsterile nasal products, and emulsions, including those applied to mucous membranes. |
|
3 |
Oral products other than antacids, made with aqueous bases or vehicles. |
|
4 |
Antacids made with an aqueous base. |
Table 2: Criteria for Tested Organisms
| Category |
Microbes |
Criteria |
|
1 |
Bacteria |
Not less than 1.0 log reduction from the initial calculated count at 7 days, not less than 3.0 log reduction from the initial count at 14 days, and no increase from the 14 days’ count at 28 days. |
|
Yeast and Molds |
No increase from the initial calculated count at 7, 14, and 28 days. | |
|
2 |
Bacteria |
Not less than 2.0 log reduction from the initial count at 14 days, and no increase from the 14 days’ count at 28 days. |
|
Yeast and Molds |
No increase from the initial calculated count at 14 and 28 days. | |
|
3 |
Bacteria |
Not less than 1.0 log reduction from the initial count at 14 days, and no increase from the 14 days’ count at 28 days. |
|
Yeast and Molds |
No increase from the initial calculated count at 14 and 28 days. | |
|
4 |
Bacteria, Yeast, and Molds |
No increase from the initial calculated count at 14 and 28 days. |

While AET covers essential Gram-positive and Gram-negative bacteria, yeasts, and molds, it does not cover all microbes. Depending on the production environment, strains like Bacillus thuringiensis, methicillin-resistant Staphylococcus aureus (MRSA), or Acinetobacter spp. may pose contamination risks. Emery Pharma can accommodate customized testing for additional organisms based on client needs. Ensuring preservatives are effective against a broad spectrum of microbes is critical for product safety.
Though preservatives extend shelf life, they do degrade over time. It is essential to observe expiration dates, which reflect the timeframe in which the preservative maintains antimicrobial efficacy. Using products like expired eye drops or mascara can result in microbial buildup, posing risks such as eye infections. This is especially critical for medical products like EpiPens, insulin, or oral nitroglycerin, which may lose potency or efficacy post-expiration. Many AET-tested microbes are opportunistic pathogens and can cause serious health concerns.
In addition to antimicrobial effectiveness testing, Emery Pharma offers a wide range of microbiology services, including antibiotic testing, resistance profiling, antimicrobial susceptibility testing and customizable biofilm model assays, tailored to meet diverse product testing needs. Contact us today to see how we can help you bring your product to market!
References
1) Moser, C. L., & Meyer, B. K. (2011). Comparison of compendial antimicrobial effectiveness tests: a review. AAPS PharmSciTech, 12(1), 222–6. doi:10.1208/s12249-010-9575-9
