Testing Antimicrobials Using Minimum Biofilm Eradication Concentration (MBEC)
Biofilm growth on rocks in a stream (USGS) and within a kitchen pipe (MSU Center for Biofilm Engineering).
Testing Antimicrobials Using Minimum Biofilm Eradication Concentration (MBEC)
Biofilm can grow nearly everywhere that microorganisms are present. They can grow on river beds, in high temperature geysers, in your kitchen sink, and even on the human body. Biofilm growth can also occur on medical devices and implants. It is commonly found on urinary and central venous catheters, pace makers, artificial heart valves, silicone breast implants, and cochlear devices.
Biofilm formation on a voice prosthesis implant.
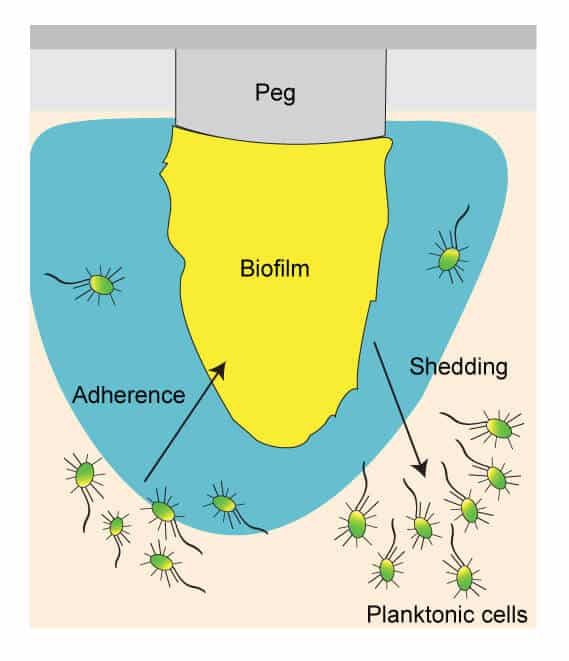
A minimum inhibitory concentration (MIC) assay is a commonly used method to test antibiotic efficacy because it is quick and reproducible. A traditional MIC is effective for testing antibiotics against free-floating, planktonic bacteria and fungi, but is not an effective assay for testing antibiotics against adherent biofilm. Biofilm is an exopolysaccharide, extracellular polymeric substance, matrix that is formed when a microbe adheres to a surface. Biofilm serves as a protective barrier that can prevent an antibiotic from killing enclosed bacteria and fungi. The antibiotic is unable to successfully penetrate the biofilm, resulting in antibiotic resistance.
Biofilm is present anywhere these microbes live, so it is important for an antibiotic, antiseptic, or disinfectant to be effective against biofilm. At Emery Pharma, we have experience and expertise in testing the efficacy of antimicrobial agents against biofilm.
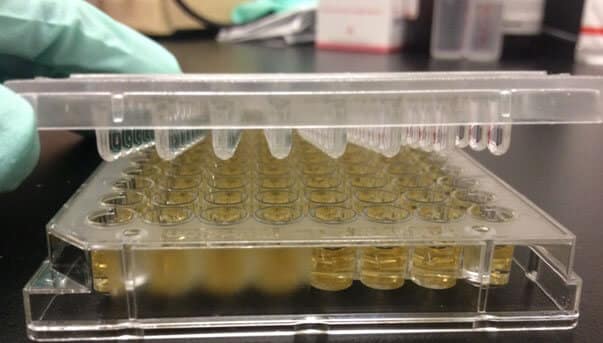
The MBEC™ (minimum biofilm eradication concentration) Assay can be used to determine the efficacy of an antimicrobial agent against biofilm. The MBEC assay uses a Calgary Biofilm Device (CBD), a 96-well plate with pegs built into the lid that allow for the adherence and growth of biofilm. MBEC assay is a very time efficient and accurate method of testing antimicrobial agent efficacy against biofilm.
Calgary Biofilm Device (CBD)
Biofilm of the desired microorganism is grown on the pegs by using the appropriate growth media and incubating in a shaker. Microorganisms that have greater motility tend to aggregate more, leading to robust biofilm formation on the pegs. Pseudomonas aeruginosa and Escherichia coli, Gram-negative bacteria which tend to be flagellated and motile, have greater biofilm density on pegs. Staphylococcus aureus, which is non-motile, is not as effective in biofilm formation. Pegs that are coated with supplemental nutrients or hydroxylapatite can be used to facilitate growth of fastidious microorganisms, such as Candida albicans.
Table 1 shows experimental biofilm densities of various organisms grown at Emery Pharma. Different species of Staphylococcus can grow different densities of biofilm. In addition, different strains of the same species have variability in biofilm densities. For example, S. aureus ATCC 29213 had a greater biofilm density than methicillin-resistant S. aureus ATCC 33591, when grown under the same conditions. EPS can grow biofilm for a wide variety of bacterial and fungal strains to fit the needs of your study.
Table 1: Organism Biofilm Densities
|
Organism |
Strain |
Average CFU/peg (n = 4) |
|
Pseudomonas aeruginosa |
ATCC 27853 |
2.83 x 107 |
|
Staphylococcus aureus |
ATCC 29213 |
1.63 x 105 |
|
Methicillin-resistant Staphylococcus aureus |
ATCC 33591 |
2.90 x 104 |
|
Staphylococcus haemolyticus |
ATCC 29970 |
6.67 x 104 |
After the biofilm is given adequate time to grow, an MBEC assay can be conducted. The Calgary Biofilm Device is rinsed to rid the pegs of planktonic microorganisms. The pegged lid is treated with serial dilutions of the antimicrobial agents. The treated pegged lid is then rinsed of excess antimicrobial agent and transferred to a plate of fresh media. The MBEC is the lowest concentration of the antimicrobial that rids the peg of biofilm, apparent if there is no growth in that well. Alternatively, a spectrophotometer can be used to find the optical density (OD) of the wells. A well is considered to have biofilm eradicated if the OD650 is less than 0.1.
Emery Pharma also offers services to determine the minimum biofilm inhibitory concentration (MBIC) of antimicrobials. The MBIC is the minimum concentration that prevents the initial formation of biofilm, while MBEC is the minimum concentration necessary to eradicate already formed biofilm. The MBIC tends to be much lower than the MBEC of an antimicrobial. This is because the concentration of antimicrobial agent needed to prevent initial formation of biofilm is much lower than the concentration necessary to eradicate already formed biofilm.
Emery Pharma has extensive experience in studies involving biofilm. We offer the MBEC™ Assay to test the efficacy of antimicrobial agents on biofilm and also offer MBIC assay services. In addition, we offer a number of other models, such as CDC reactor, drip-flow reactor, capillary biofilm, and can help with your custom biofilm study needs. Please contact EP if you are interested in our biofilm services.
References:
1. Ceri H, Olson ME, Stremick C, Read RR, Morck D, Buret A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. Journal of Clinical Microbiology 1999; Vol 37, No. 6: p. 1771-1776.