Understanding Antimicrobial/Preservative Effectiveness in Everyday Products
Toothpaste, lotions, cosmetics, eye drops and medications are just some of the products that we all use on a daily basis. Unfortunately, there are two ways that these products can get contaminated; it can either occur during the manufacturing process or during usage. During usage, contamination occurs when the product is constantly opened and closed. These products are potentially exposed to many types of microbes, from airborne fungal spores to the bacteria on your fingertips. These microbes need few nutrients to grow and thrive. To prevent any microbes from taking advantage of the environment, preservatives are added to these products to prevent the growth and protect the longevity of the product. Preservatives are also commonly found in many food and beverage products. Some of the common preservatives found in products are benzalkonium chloride, chlorhexidene, sodium benzoate, thimerosal, sorbic acid, citrate acid and benzoic acid. These preservatives create an antimicrobial environment.
To test the preservatives’ effectiveness, an assay was developed, United States Pharmacopeia-51 (USP-51), more commonly known as the Antimicrobial Effectiveness Test (AET). The AET investigates if common microorganisms will flourish or die off in these products over time. This test utilizes: Aspergillus brasiliensis ATCC 16404 (a common black mold that causes plant disease), Candida albicans ATCC 10231 (a commensal yeast that can be invasive to immunocompromised patients), Escherichia coli ATCC 8739 (part of the gut flora that can cause a lot of harm), Pseudomonas aeruginosa ATCC 9027 (found in most environments but can cause moderate to severe infections), and Staphylococcus aureus ATCC 6538 (known for causing skin lesions and food poisoning).
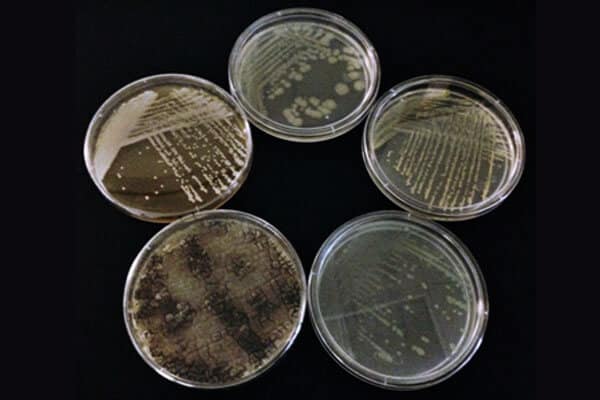
Figure 1: (clockwise from the top) Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6538, Pseudomonas aeruginosa ATCC 9027, Aspergillus brasiliensis ATCC 16404, and Candida albicans ATCC 10231 grown in the EP Microbiology Lab
AET quantitatively assesses the effectiveness of a preservative in microbial growth prevention. Emery Pharma (EP) performs AET following the USP-51, European Pharmacopeia-5.1.3 and Japanese Pharmacopeia-19 methods. A combined method that satisfies all Pharmacopeia requirements was recently described (Moser 2011). Briefly, the inoculum should not exceed 1% of the testing agent and the test should be incubated at 35°C for bacteria and 25°C for fungi. Time points according to the U.S. Pharmacopeia and Japanese Pharmacopeia shall be 7, 14 and 28 days, while the European Pharmacopeia also includes a 6 and 24 hour time point. Additional time points may be agreed upon depending on how swiftly the preservative may work and for how long. At each time point, aliquots will be removed, serially diluted and plated onto appropriate agar, such as Soybean-Casein Digest Agar for bacteria and Sabouraud Dextrose Agar for fungi to determine cell viability.
In order for the preservative to pass the AET, results must show A. brasiliensis and C. albicans not exceeding the original inoculum. However, bacteria must have a 1 to 3 log reduction, depending on the product’s usage and description found in Table 1 and 2 from USP-51.
Table 1: Compendial Product Categories
|
Category |
Product Description |
|
1 |
[Injectables], other parenterals including emulsions, otic products, sterile nasal products, and ophthalmic products made with aqueous bases or vehicles. |
|
2 |
Topically used products made with aqueous bases or vehicles, nonsterile nasal products, and emulsions, including those applied to mucous membranes. |
|
3 |
Oral products other than antacids, made with aqueous bases or vehicles. |
|
4 |
Antacids made with an aqueous base. |
Table 2: Criteria for Tested Organisms
| Category |
Microbes |
Criteria |
|
1 |
Bacteria |
Not less than 1.0 log reduction from the initial calculated count at 7 days, not less than 3.0 log reduction from the initial count at 14 days, and no increase from the 14 days’ count at 28 days. |
|
Yeast and Molds |
No increase from the initial calculated count at 7, 14, and 28 days. | |
|
2 |
Bacteria |
Not less than 2.0 log reduction from the initial count at 14 days, and no increase from the 14 days’ count at 28 days. |
|
Yeast and Molds |
No increase from the initial calculated count at 14 and 28 days. | |
|
3 |
Bacteria |
Not less than 1.0 log reduction from the initial count at 14 days, and no increase from the 14 days’ count at 28 days. |
|
Yeast and Molds |
No increase from the initial calculated count at 14 and 28 days. | |
|
4 |
Bacteria, Yeast, and Molds |
No increase from the initial calculated count at 14 and 28 days. |
AET covers the basic Gram-positive and Gram-negative bacteria, along with yeast and mold strains. Unfortunately, there may be other microbial strains resistant to the preservative that are not being tested against. Depending on the manufacturing environment or usage, other strains such Bacillus thuringiensis, Methicillin-resistant Staphylococcus aureus or Acinetobacter spp. can contaminate samples or a production run. EP can accommodate such additional testing needs. It is necessary that the preservatives used in the products are capable of keeping all types of microbes away.
Although preservatives increase the shelf life of products, it does not mean that it will work indefinitely. Therefore it is important to follow expiration dates, because these dates reflect approximately the amount of time it takes before the preservatives start breaking down, allowing for microbes to take advantage and accumulate. After the expiration dates, it is not recommended to use any of the products due to the lack of antimicrobial efficacy. For example, expired opened eyedrops or mascara sitting on your bathroom counter could have accumulated a significant microbial load that potentially cause an eye infection. It is especially not recommended to have an EpiPen, insulin, or oral nitroglycerin after their expiration dates, because these products do lose potency and should be used at the appropriate strength. Most of the microbes used for the AET are opportunistic pathogens and may be detrimental to one’s health.
Besides the antimicrobial effectiveness test, Emery Pharma also offers a wide variety of additional microbiology services including antibiotic testing, resistance profiling services, antimicrobial susceptibility testing and a variety of biofilm models all of which can be adapted to suit any testing needs.
References
Moser, C. L., & Meyer, B. K. (2011). Comparison of compendial antimicrobial effectiveness tests: a review. AAPS PharmSciTech, 12(1), 222–6. doi:10.1208/s12249-010-9575-9