Background
N-Nitrosamines are a class of chemical compounds with the general structure R1R2N–N=O; R1=R2= Me = NDMA. The FDA has identified seven nitrosamine impurities that theoretically could be present in drug products. Five of them (NDMA, NDEA, NMBA, NIPEA, and NMPA) have been detected in drug substances or drug products.
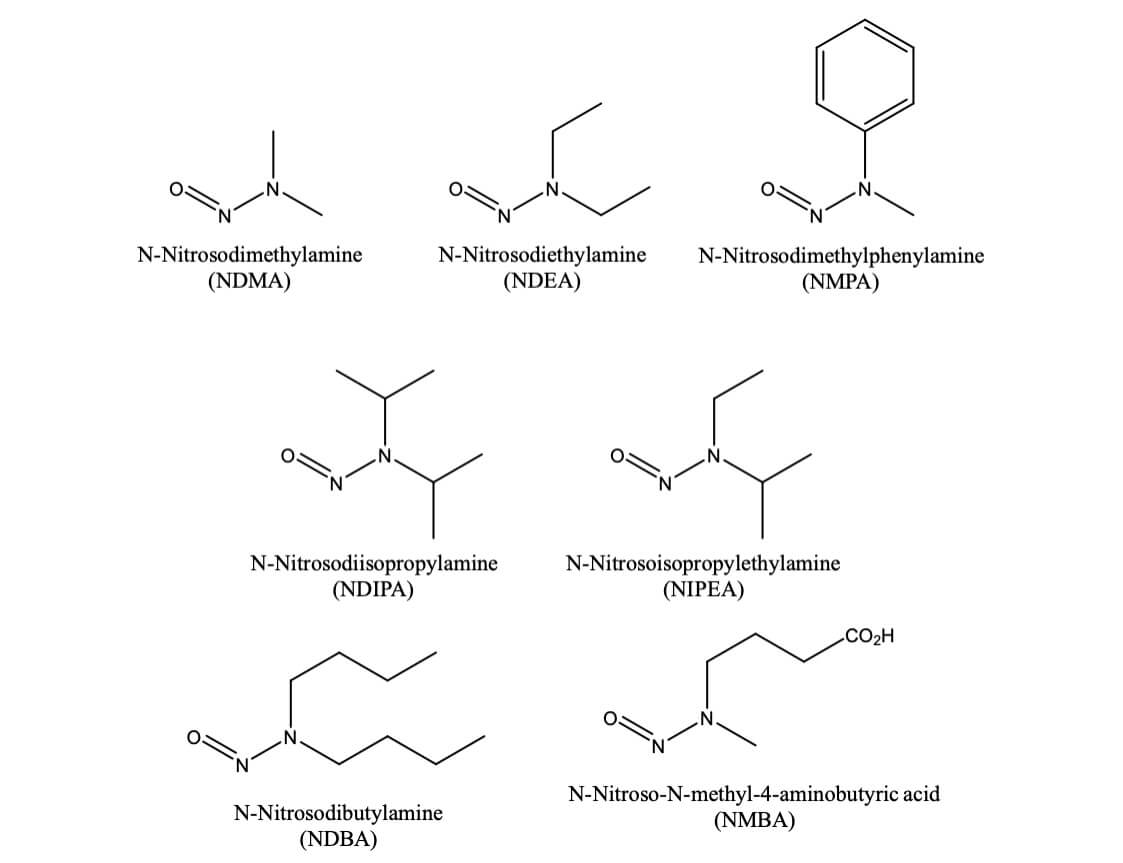
According to the ICH M7 Guideline, nitrosamines are classified as probable human carcinogens and included as part of the “cohort of concern” due to their carcinogenic risk. Nitrosamine impurities have been found at unacceptable levels in several drug products including ARBs (-sartans), ranitidine, nizatidine, and metformin. Recently, Nitrosamine impurities have been detected in Merck’s blockbuster Diabetes drug, Januvia (Sitagliptin).
In April 2020, Emery Pharma was responsible for identifying the root cause of NDMA formation in ranitidine (Zantac). We filed a citizen petition to the FDA regarding our findings. As a result, the FDA issued a nationwide recall, see more.
Analytical Procedures for Quantitation:
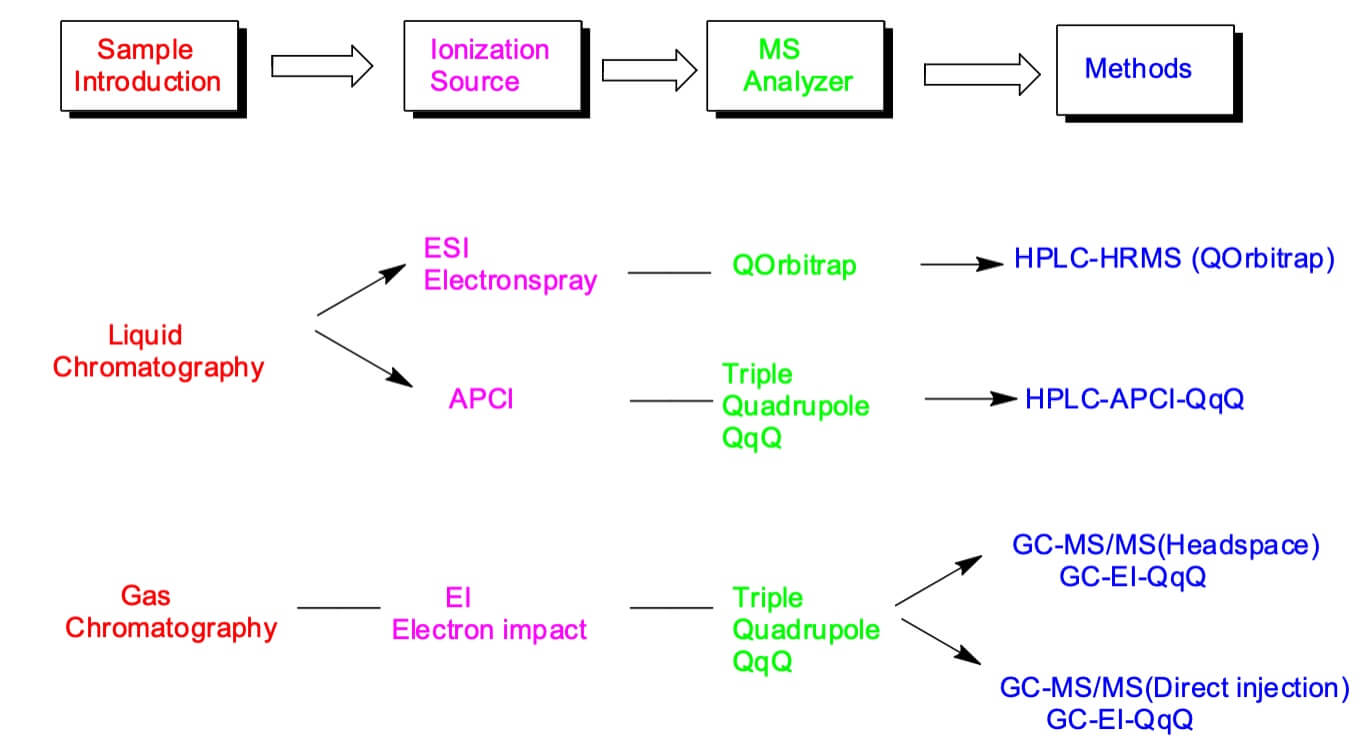
Sensitive analytical methods with limits of quantitation (LOQ) in the parts-per-billion (ppb) range may be needed. The FDA recommends using methods with LOQs at or below 0.03 ppm. Several methods using liquid chromatography tandem MS (LC-MS/MS) and gas chromatography electron impact (triple quadrupole, QqQ) were developed to detect both thermally stable and unstable nitrosamines.
FDA Methods:
In 2018, health authorities in the European Union, the United States, Canada, and other countries began investigating the presence of N-nitrosamine impurities in medicines. Initially, N-nitrosodimethylamine (NDMA) was identified in certain antihypertensive drugs then, health authorities have identified N-nitrosamines in several other categories of drugs, including in common heartburn products (ranitidine, nizatidine), in antidiabetic drugs (metformin), and, more recently, in medicines used to treat and prevent tuberculosis (rifampicin, rifapentine). The “acceptable level” of N-nitrosamines is defined by a measure called acceptable intake (AI). The method for determining AI is set by the internationally recognized ICH M7 (R1) guideline (1). The ICH M7(R1) recommends calculating a compound-specific AI based on rodent carcinogenicity potency data such as TD50 values (doses giving a 50% tumor incidence in rodents). Once calculated, the AI is converted into a measure of parts per million (ppm). The conversion of AI into ppm varies by product and is calculated based on a drug’s maximum daily dose (MDD) as reflected in the drug label: AI (ppm) = AI (ng) / MDD (mg). Converting AI into ppm gives a measure of acceptable N-nitrosamine concentration in drug substance, which can be monitored by manufacturers and regulators. Detection methods include solid phase extraction, gas chromatography and liquid chromatography. The analytical methods have been developed to quantify NDMA in the desired precursors. The several methods using liquid chromatography tandem MS (LC/MS/MS) and gas chromatography electron impact (triple quadrupole, QqQ) were developed to detect both thermally stable and unstable nitrosamines.
The FDA recommends methods utilizing LC-ESI-HRMS, GC-MS/MS, LC-APCI-QqQ (6-10) for quantitation.
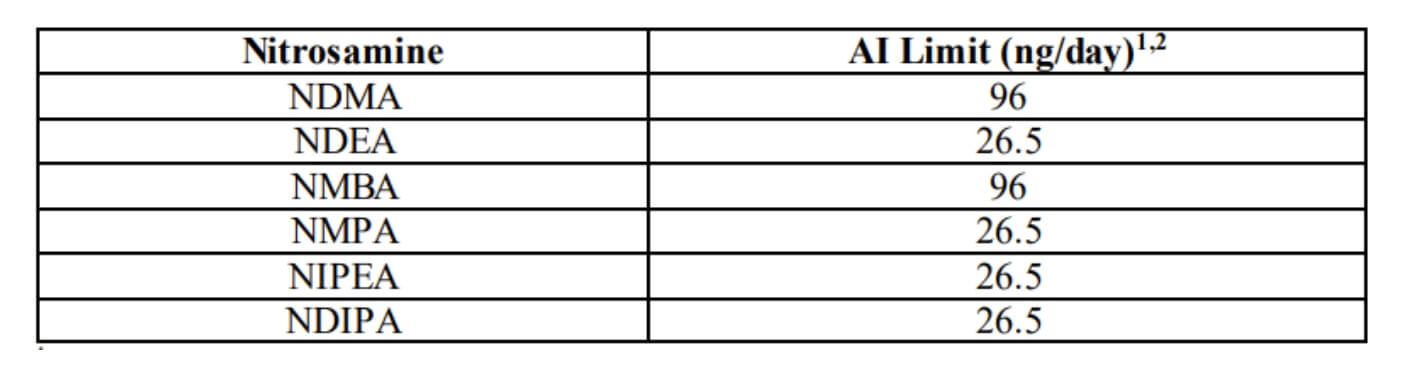
Acceptable Intake Limits Outlined by the FDA:
Potential Root Causes of Nitrosamine Impurities in Drugs:
- Presence of secondary, tertiary, or quaternary amines and nitrite salts under acidic reaction conditions
- Use of sodium nitrite (NaNO2), or other nitrosating agents
- Contaminants present in raw materials during API manufacturing
- Recovered Solvents, Catalysts, and Reagents as Sources of Contamination
- Cross-contamination
- Quenching Process as a Source of Nitrosamine Contamination
- Lack of Process Optimization and Control
- Nitrites used as reagents in one step can carry over into subsequent steps
- Degradation processes/stability: This could potentially occur in starting materials, intermediates and drug substances as well as during finished product formulation or storage.
New FDA Guidelines:
Drug manufacturers must report changes implemented to prevent or reduce nitrosamine impurities in accordance with FDA regulations (21 CFR 314.60, 314.70, 314.96, and 314.97).
Pre-submission: FDA recommends that applicants conduct a risk assessment for nitrosamine impurities in APIs and proposed drug products and conduct confirmatory testing prior to submission of an original application, or file an amendment as needed.
Applications Pending With the Agency: Applicants with pending applications should conduct a rapid risk assessment and inform FDA testing finds nitrosamine levels above the Acceptable Intake limit.
References:
- European Medicines Agency. ICH M7 assessment and control of DNA (reactive) mutagenic impurities in pharmaceutical to limit potential carcinogenic risk. Amsterdam: European Medicines Agency; 2018. https://www.ema.europa.eu/en/ich-m7-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit-potential#current-effective-version–section.
- EPA 2004. “U.S. EPA Method 521: Determination of Nitrosamines in Drinking Water by Solid Phase Extraction (SPE) and Capillary Column Gas Chromatography with Large Volume Injection and Chemical Ionization Tandem Mass Spectrometry (MS/MS).” Version 1.0. National Exposure Research Laboratory, Cincinnati, Ohio. EPA 600-R-05-054
- EPA. 2007. Provisional Peer Reviewed Toxicity Values for N-Nitrosodimethylamine (CASRN 62- 75-9). 6-19-2007.
- U.S. Environmental Protection Agency (EPA).1996. “Method 8070A. Nitrosamines By Gas Chromatography.”
- EPA. 1998. “Methods 8270D. Semi-volatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS).”
- U.S. Food & Drug Administration (FDA). Combined Direct Injection NNitrosodimethylamine (NDMA), N-Nitrosodiethylamine (NDEA), NNitrosoethylisopropylamine (NEIPA), N-Nitrosodiisopropylamine (NDIPA), and N-Nitrosodibutylamine (NDBA) Impurity Assay by GC-MS/MS. 2019a. https:// www.fda.gov/media/123409/download. Accessed 19 Apr 2019.
- U.S. Food & Drug Administration (FDA). Combined Headspace N-Nitrosodimethylamine (NDMA), N-Nitrosodiethylamine (NDEA), NNitrosoethylisopropylamine (NEIPA), and N-Nitrosodiisopropylamine (NDIPA) Impurity Assay by GC-MS/MS. 2019b. https://www.fda.gov/media/124025/ download. Accessed 29 Apr 2019.
- U.S. Food & Drug Administration (FDA). Development and validation of a RapidFire-MS/MS method for screening of nitrosamine carcinogen impurities N-Nitrosodimethylamine (NDMA), N-Nitrosodiethylamine (NDEA), N-Nitrosoethylisopropylamine (NEIPA), N-Nitrosodiisopropylamine (NDIPA), N-Nitrosodibutylamine (NDBA) and N-Nitroso-N-methyl-4-aminobutyric acid (NMBA) in ARB drugs. 2019c. https://www.fda.gov/media/125477/ download. Accessed 24 July 2019.
- U.S. Food & Drug Administration (FDA). FDA updates table of interim limits for nitrosamine impurities in ARBs. 2019d. https://www.fda.gov/drugs/drugsafety-and-availability/fda-updates-and-press-announcements-angiotensin-ii-receptor-blocker-arb-recalls-valsartan-losartan#interimlimits2. Accessed 28 Feb 2019.
- U.S. Food & Drug Administration (FDA). Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS) Method for the determination of six nitrosamine impurities in ARB drugs. 2019e. https://www.fda.gov/media/1254 78/download. Accessed 21 May 2019