Arrest Bacterial Communication: Interfering with Quorum Sensing to Inhibit Biofilm Formation
Are you hesitant to enter the hospital emergency room, fearing that you may get infected with deadly bacteria? Well, this is something many of us are concerned about these days. The threat of multidrug resistant bacteria, commonly referred as ‘Superbugs’, is increasing day by day, and a recent episode of disaster was reported in Nevada, USA where a woman died because of untreatable medical condition caused by a Klebsiella pneumoniae superbug.
This multidrug resistant K. pneumoniae is a Gram-negative bacterium grouped as CRE — carbapenem-resistant enterobacteriaceae, and often causes severe urinary tract infections. The infection was so severe that it became a systemic blood borne infection. What was alarming was the fact that this pathogen was resistant to 26 different antibiotics. Clinicians and scientists believe that the multidrug resistant bacteria could cause 10 million deaths per year by 2050 if left unchecked. Thus, there is a growing concern about treating multidrug resistant bacteria [1].
It is interesting to note that the all multidrug resistant bacteria can form biofilms which make them extremely difficult to treat. In bacterial biofilms, bacteria live in cooperative communities embedded in a self-produced extracellular matrix. Quorum sensing (QS) is phenomena by which the bacteria communicates with each other and acts as a community. QS plays an important role in biofilm formation. QS regulate the target gene expression responsible for the phenotypes essential to pathogenicity/symbiosis through autoinducer signaling molecules. In Gram-negative bacteria, QS is mediated through an autoinducer signaling molecule called, N-acyl homoserine lactones (AHL). QS controls the production of virulence factors such as exopolysaccharide synthesis, biofilm formation, swarming motility, pigment and toxin production in some pathogenic bacteria. Because of the rapid emergence of bacterial antibiotic resistance and the importance of QS during bacterial pathogenesis, interfering with the mechanism of QS is an attractive alternative strategy to attenuate bacterial virulence. QS inhibitory compounds, unlike conventional antibiotics, do not kill or inhibit microbial growth and are less likely to impose a selective pressure for the development of drug resistant bacteria. Recent studies have identified that a few natural products including plant extracts have properties for modulating bacterial QS system, thereby reducing the bacterial virulence [2-4].
Different strains of bacteria can be used to study quorum sensing and one of the most commonly used microorganism is Chromobacterium violaceum (C. violaceum).
C. violaceum is a Gram-negative bacterium that produces the purple-colored pigment violacein in response to QS regulated gene expression mediated by its autoinducer, AHL. C. violaceum is a simple and efficient model microorganism for studying QS. The wild-type C. violaceum strain, always expresses the autoinducer, AHL and produce purple pigment on agar media whereas the biosensor strain, C. violaceum CV026 (mini-Tn5 mutant of wild type strain) deficient in autoinducer synthase produces purple pigment only when AHL is added exogenously in the agar media.
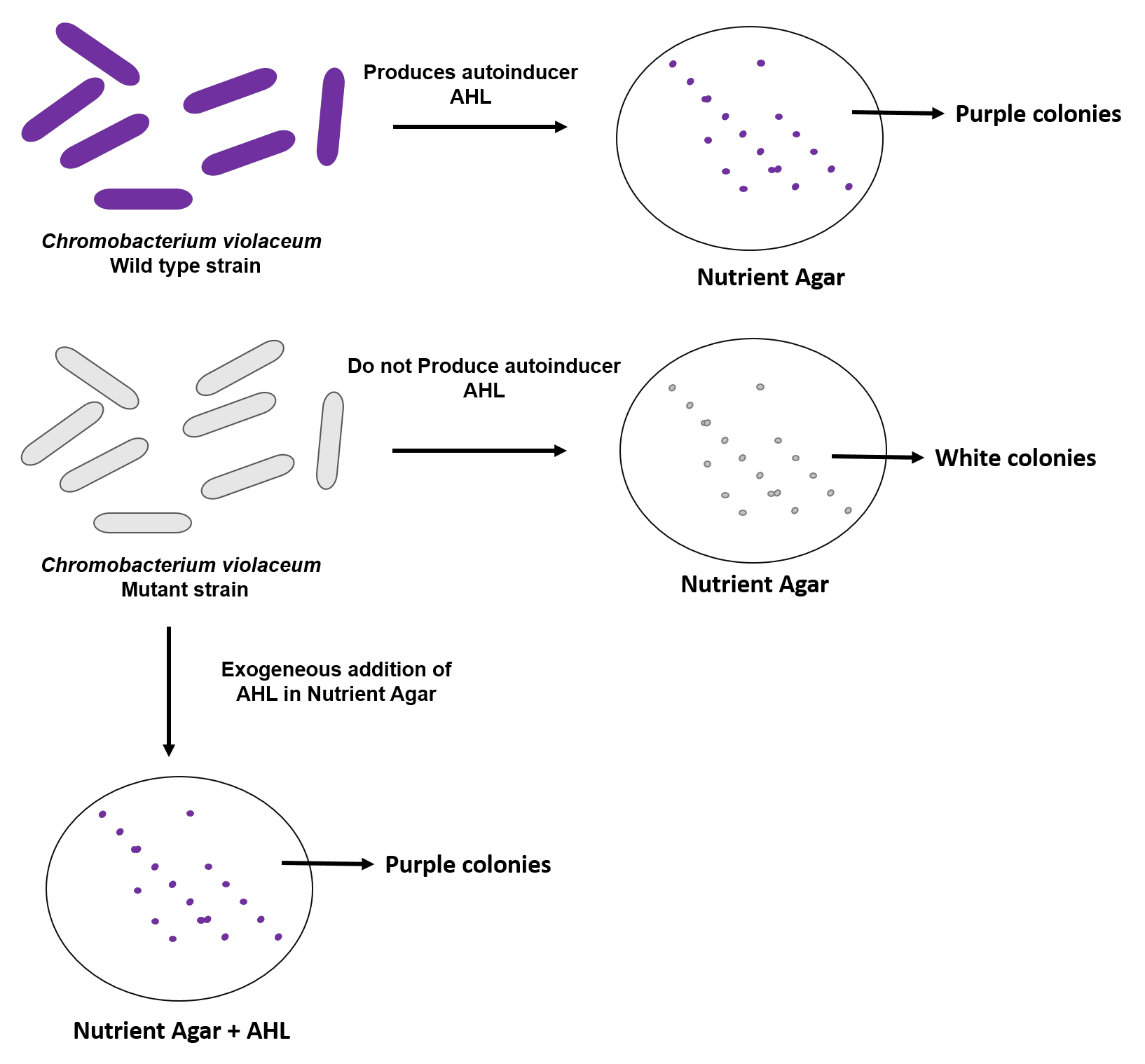
Both the organisms have been successfully used for studying various QS mechanisms, and they offer a convenient tool for the identification of QS inhibitors/antagonists. At Emery pharma, we have established assays for the identification of QS inhibitors or antagonists in plant extracts using wild type and mutant strain of C. violaceum. If the plant extracts or test article produces QS inhibitors or antagonists, there will be inhibition of production of the purple-colored pigment violacein resulting in a zone of colorless around the plant fragment or the disc containing the test article.
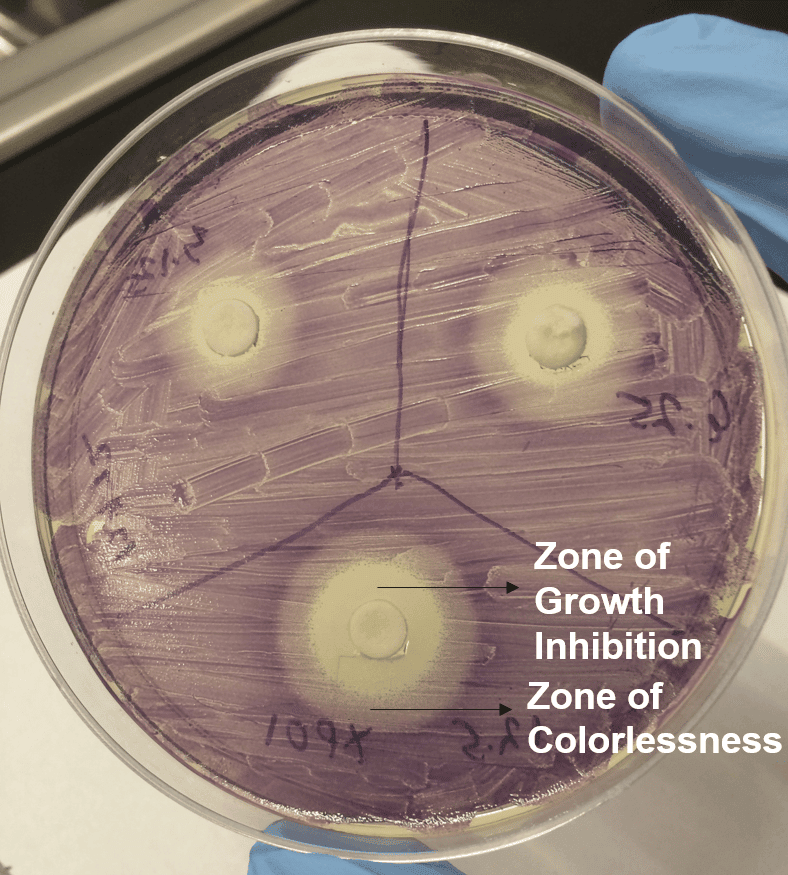
The image on the left is a representative data from a recent study where we performed QSI assay. The data shows zone of growth inhibition and zone of colorlessness induced by a plant based test article. At sub-lethal concentration, the production of purple pigment by C. violaceum is inhibited (zone of colorlessness) which is indicative of QSI.
Emery pharma offers QSI assays and other microbiological testing services. Emery Pharma uses the expertise of our Ph.D. and MSc scientists not only in performing tests for clients, but utilizing their knowledge to help figure out the best mode of action to obtain the results needed, help answer any questions along the way, and provide expert analysis.
About the author:
Sridhar Arumugam holds a Ph.D. degree in Microbiology and he is the Director of Cell and Microbiology with Emery Pharma.
References
- Kostyanev, T., Bonten, M.J. and Goossens, H., 2017. Anti-infectives and the Lung: ERS Monograph 75, 75, p.289.
- McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW, Stewart GS. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997 Dec 1;143(12):3703-11.
- Vasavi HS, Arun AB, Rekha PD. Inhibition of quorum sensing in Chromobacterium violaceum by Syzygium cumini L. and Pimenta dioica L. Asian Pacific journal of tropical biomedicine. 2013 Dec 31;3(12):954-9.
- McLean RJ, Pierson LS, Fuqua C. A simple screening protocol for the identification of quorum signal antagonists. Journal of Microbiological Methods. 2004 Sep 30;58(3):351-60.
