Blood: Sample Analysis
Every drop of blood is a snapshot of our health. From the genetic makeup of our genes we are actively expressing, the concentration of metabolites in our body, to the levels of our immunity to various toxins and pathogens, blood can provide all this information and more. (1)
But to acquire this wealth of data you have to know how to handle that drop! The purpose of this blog is two-fold:
- To aid in making the right choice of blood sample to collect depending on what you want to measure, and
- How to handle blood samples once collected
Blood samples and blood products can be potentially hazardous and/or infectious. When handling blood, always wear the proper protective garments to protect yourself and those around you, including eye protection, laboratory gloves, and a laboratory coat. It is also a good idea to make sure you are up to date with your immunization shots, including hepatitis B.
First, blood in circulation (in vivo) is considered whole blood, i.e., it contains all the cellular and liquid components necessary for its normal function. Whole blood can be collected (or ‘drawn’) into a tube with anticoagulants to prevent the blood from clotting, or into a tube without anticoagulants, in which case the blood will clot (see Figure 1).
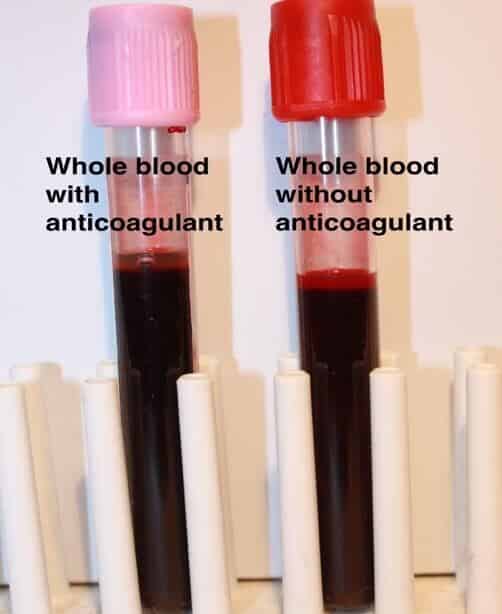
FIGURE 1: Note that there is no difference in the way the blood appears immediately after being collected. The only difference is in the color of the caps of the tubes:
Pink = anticoagulant (for this color, EDTA)
Red = clotting tube (no anticoagulant)
In the clotting tube (red cap), the clot will form over a period of 30-60 minutes at room temperature, after which the tube can then be centrifuged. After centrifugation, what remains is a clear, straw-colored liquid on top of a dark red clot (the clumped blood cells tangled in the fibrin mesh). This straw-colored, acellular liquid is called serum (see Figure 2).

FIGURE 2: Serum – the acellular fraction of blood that has been allowed to clot. Clotting tubes are always centrifuged to ‘spin down’ the clot. The serum can then be removed by pipetting, then stored and used for later analyses.
Please note: “Allowed to clot” means without disturbing the tube of blood during formation of the clot. Do not shake a tube of blood and never vortex a tube of blood. Strong agitation will cause the red blood cells (RBCs) to break open (hemolyze) and release hemoglobin, turning the straw-colored fluid into various shades of red. A very slight reddish tinge is usually acceptable, but moderate to dark red color indicates severe hemolysis. If a serum sample is this color, it may not be suitable for further analyses because the release of hemoglobin into serum will falsely elevate protein levels, and, because hemoglobin is a ‘sticky’ protein, it can interfere with spectrophotometric measurements as well as antigen-antibody interactions. If you must examine the blood tube for the progress and/or presence of clot formation, gently rock the tube back and forth (this is true for any blood in any tube). (2)
What can serum be used to analyze?
Serum is the first choice for chemistry panels, i.e., the measurement of salts (e.g., sodium, potassium), proteins (e.g., cholesterol, bilirubin), and other metabolites in the blood. Serum is also used to measure drug levels, e.g., for pharmacokinetic studies whereby a drug is administered to a subject after which the subject’s blood is sampled over time to measure the clearance of the drug (how much drug is left in the blood over time). In immunology and infectious diseases, serum is used to measure the level of foreign agents (i.e., antigens) such as toxins or infectious organisms, and the subsequent immune response (i.e., antibodies) to these foreign agents.
In contrast, if we want to study the blood’s cellular morphology, we cannot use clotted blood as all the RBCs and white blood cells (WBCs) are enmeshed in the clot. Therefore, the blood must be collected into a tube containing anticoagulant. Common anticoagulants include EDTA, sodium heparin, and acid-citrate-dextrose (ACD). The color of the tube’s cap indicates the anticoagulant inside. When blood is drawn into a tube containing anticoagulant it remains in liquid form, free of any clot formation. When we centrifuge this tube, the result is a slightly cloudy, straw-colored liquid on top of what is commonly referred to as ‘packed cells’ (as opposed to clotted cells). Because they are not clotted, packed cells can be easily re-suspended by gently rocking the blood tube to mix the cells with the plasma (see Figure 3).

FIGURE 3: Plasma – the acellular liquid fraction of whole blood containing anticoagulants to prevent a clot from forming. Note that unlike the clot, the packed cells can be easily resuspended by gentle agitation. Care should be taken to remove the plasma without disturbing the blood cells.
Plasma contains all the components found in serum, and also contains all the clotting factors and fibrin precursors. Plasma can be used for almost any assay or measurement that serum is used for (e.g., pharmacokinetic assays). There are some limitations, however, so it is best to investigate before choosing to collect plasma instead of serum. Furthermore, depending on the downstream application, anticoagulants are not interchangeable: for example, residual amounts of sodium heparin can inhibit the enzyme reverse transcriptase and interfere with RT-PCR.
Below the plasma layer are intact RBCs and WBCs that can be used to evaluate cellular morphology, e.g., in a routine, multi-parameter test called a complete blood count (CBC) that examines for diseases such as anemia and cancer. The WBCs can be further separated from the RBCs for use in cell culture, as well as various live-cell assays such as flow cytometry, cytotoxic T-cell assays, and cytotoxicity assays. Here again, the choice of anticoagulant is important depending on the downstream application: Acid-Citrate-Dextrose (ACD) causes crinkling (crenellation) of RBC membranes and thus is not a suitable anticoagulant when examining RBC morphology, such as in a CBC analysis.
What blood sample is required for molecular-based analyses such as next-generation sequencing (NGS) of DNA and RNA?
For the analysis of intracellular DNA, either from WBCs or from circulating fetal or tumor cells, blood is collected into tubes containing a routine anticoagulant as a starting point. Extracellular DNA (often referred to as circulating DNA or cell-free DNA) can also be measured from the plasma fraction from these same tubes. However, as WBCs will break open (lyse) over a period of days, if cell-free DNA is to be measured, the WBCs must be stabilized so as not to lyse and contaminate the cell-free pool of DNA. An example of one such blood collection tube is manufactured by Streck (3) that contains a preservative to stabilize WBCs for up to seven days at room temperature.
RNA is a more delicate analyte. Intracellular levels of RNA can change within minutes after the blood leaves the body, and extracellular (cell-free) RNA requires immediate protection from degradation. An example of a blood tube used to stabilize RNA for NGS is the PAXgene Blood RNA tube (4). The blood is drawn directly into this tube and then gently mixed. The RNA is stable for up to seven days before extracting for sequence analyses.
Finally, some general rules about protecting your samples:
- For serum & plasma: Process blood tubes as soon as possible, and store serum and plasma frozen and ship frozen. If delays are unavoidable (e.g., the tube of blood must be shipped to a laboratory for processing), refrigerate the tubes (but do not freeze!) until shipment can be arranged and/or centrifugation can be performed
- For live cells from whole blood: Store blood at room temperature and harvest the WBCs within 24 hours if possible
- DO NOT REFRIGERATE the blood tubes. Refrigeration can cause WBCs to modulate their surface receptors. Many years ago, RAIDS or ‘refrigerator-induced AIDS’ was a common clinical artifact as low temperatures cause CD4 cells to retract their receptors, falsely changing CD4/CD8 ratios
- NEVER FREEZE WHOLE BLOOD TUBES. The cells will lyse and upon thawing you will wind up with a gooey mess
- For DNA and RNA: For DNA, routine anti-coagulated blood will often suffice. Special considerations should be made for sequencing cell-free DNA. RNA needs to be protected and special blood tubes are required.
Here at Emery Pharma we routinely analyze serum and plasma to detect specific chemicals and pharmaceuticals, e.g., to measure the pharmacokinetics of a new drug. We also examine serum and plasma for specific antigens and antibodies, including viral antigens and cytokines, and develop customized EIAs for various analytes. For cell-based assays we have a variety of human and animal cell lines, both primary and transformed, for use as targets or for co-culturing. Please contact us at https://emerypharma.com/contact/.
About the author:
Charles Vincent Herst, Ph.D., MPH, has worked in research and clinical laboratories around the world and is a senior scientist at Emery Pharma in Alameda, CA. He also holds a Clinical Laboratory Supervisor’s license in the State of Nevada.
References:
- Heinz-Diehl, K., et al. A Good Practical Guide to the Administration of Substances and Removal of Blood, Including Routes and Volumes. J Applied Toxicology 21: 15-23 (2001).
- UK Biobank – Blood Sample Collection, Processing and Transport, Version 1.0 http://www.ukbiobank.ac.uk/ 15 April 2011
- https://www.streck.com/collection/cell-free-dna-bct/
- http://www.bdbiosciences.com/us/applications/blood-collection/cell-biomarker-preservation/paxgenereg-blood-rna-tube/p/762165