How to Talk like a Medicinal Chemist in 10 minutes
I have worked in the drug discovery area for decades, and I often find that scientists, new or outside the field, are very confused by the terminology used by medicinal chemists. Below is a quick introduction to the vocabulary used on a daily basis in pharmaceutical research and development, to get you talking like a drug development professional in no time!
The drug development process involves the interaction of many groups of scientists to ultimately bring a therapeutic candidate from an idea to FDA approval. This includes researchers such as physicians, chemists, biologists, intellectual property (IP) attorneys, pharmacologists, and regulatory personnel. Since these cross-functional groups need to interact efficiently, each person needs to understand the terminology used by other groups. As an example, a medicinal chemist needs to understand the language used by computational chemists, analytical chemists, and process chemists, as well as be well-versed in biology, intellectual property law, and pharmacology.
The process of development starts with Discovery. Discovery is where novel chemical compounds with biological activity are identified. This biological activity may result from interaction with a specific enzyme or with an entire organism. These identified Hits are often found during the screening of chemical libraries, computer simulations, or screening of naturally isolated materials, such as from plants, bacteria, and fungi. A Hit Confirmation is then conducted to verify that the compound is interacting with its intended biological target.
Hit to Lead, also called Lead Generation, is the process of chemically modifying the hit molecule to improve its activity towards a specific biological Target(s) while reducing undesired biological traits (such as toxicity and side effects). Modified hits, where the chemical structure has been systematically varied, are called Analogs. Synthesizing analogs of a “Hit” is referred to as Hit Expansion. Medicinal chemists synthesize these analogs in the lab, using well-established organic chemistry techniques. To increase synthetic throughput, chemists often focus on a specific reaction or set of reactions to assemble Building Blocks together to make a series of analogs quickly. “Building Block” is a term used to describe a compound that possesses both a reactive functional group (needed to bond with another building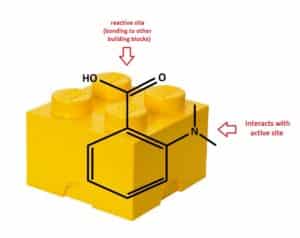 block) and an atom(s) that can interact with the Active Site. The active site is a region on the target where the analog (Substrate) binds. The interaction can be Van der Waals, π-stacking, ionic, polar, and hydrogen bonding. The binding in the active site can either follow the Lock and Key Model, or the Induced Fit Model. In the Lock and Key Model, the analog fits perfectly in the active site with no need for the enzyme to change its shape (Conformation). In the Induced Fit Model, the analog and active site are not exactly complementary, and the flexible active site needs to change its shape until the analog is bound. X-ray Crystallography is used to determine the 3D Molecular Structure of an enzyme. The 3D Coordinates of each atom in the enzyme are fed into Computational Drug Design Software, which allows a medicinal chemist to design analogs that optimize interactions (binding affinities) with the active site.
block) and an atom(s) that can interact with the Active Site. The active site is a region on the target where the analog (Substrate) binds. The interaction can be Van der Waals, π-stacking, ionic, polar, and hydrogen bonding. The binding in the active site can either follow the Lock and Key Model, or the Induced Fit Model. In the Lock and Key Model, the analog fits perfectly in the active site with no need for the enzyme to change its shape (Conformation). In the Induced Fit Model, the analog and active site are not exactly complementary, and the flexible active site needs to change its shape until the analog is bound. X-ray Crystallography is used to determine the 3D Molecular Structure of an enzyme. The 3D Coordinates of each atom in the enzyme are fed into Computational Drug Design Software, which allows a medicinal chemist to design analogs that optimize interactions (binding affinities) with the active site.
After analogs are synthesized, the biologists conduct Biological Screening of these analogs. Among other things, biochemists look at the Enzyme Activity, which shows how well the analogs bind to the active site. Generally, the better the binding affinity (analog–active site attraction), the better the biological activity, though not always. Additionally, they may also examine off-target binding to undesired targets, to predict how toxic the compound may be. Biologists test in vitro (outside of a living organism) for Whole-Cell Activity, which assesses the activity of the analog at the cellular level. They also test on Human Cell Lines, which assesses the toxicity of the analog.
After the screening, a meeting with medicinal chemists, computational chemists, cell biologists, microbiologists, biochemists, and IP attorneys usually takes place to evaluate the next step. At this point, additional analogs are proposed, and the whole drug optimization process starts again. The analogs are optimized when all parameters are met. This is when the analogs are evaluated in vivo (in a living organism), usually in mice, to test for whole-animal efficacy.
Medicinal chemists, biologists, and IP attorneys work on assessing the Freedom to Operate (FTO) landscape. This analysis determines whether commercializing a product can be done without infringing on the intellectual property rights of others. If there are no patents claiming a specific idea as Intellectual Property (IP), a Utility Patent is filed to protect that idea for 20 years. This can be a Composition of Matter Patent, where specific compound(s) are claimed. Alternatively, a Process Patent is filed, which claims a synthetic route for making specific compounds.
The collective preclinical data will provide guidance to upper management, and a decision is made whether the lead compound advances to the clinic or the whole project is canceled (known as the GO/NO GO Decision). If the decision is a “GO,” the Lead Compound is advanced into Clinical Trials and tested in humans. The goal is to get it through the clinic and obtain FDA approval. At that time, the new drug can be sold to the general population.
Understanding the terminology of drug discovery and medicinal chemistry is essential for effective communication and collaboration in pharmaceutical research. With this foundational knowledge, you’ll be better equipped to navigate the complexities of preclinical development, lead optimization, and intellectual property strategy! If you’re looking for expert support in medicinal chemistry, assay development, or pharmaceutical analysis, Emery Pharma is here to help. Contact us today to learn how our scientific expertise can accelerate your drug discovery program!
About the Author
Originally authored by Dr. Charles Francavilla. This article was reviewed and updated on June 10, 2025 by Dr. Ron Najafi, Emery Pharma's CEO.
