Native Mass Spectrometry (Native-MS) for Aggregate and Charge Variant Analysis
Size exclusion chromatography (SEC) and ion exchange chromatography (IEX) are traditional methods utilized for characterizing biotherapeutics under non-denaturing conditions. The typical high salt concentrations in their mobile phases make them incompatible with mass spectrometry (MS) detection. Native-MS stands out as a cutting-edge technique for the analysis of aggregates and charge variants in biomolecules. This approach involves introducing biomolecules into the gas phase while maintaining their native, non-denatured conformations and preserving their interactions. Unlike traditional methods, Native-MS avoids the use of denaturing solvents that might alter the structural integrity of the molecules. This methodology proves particularly valuable in the characterization of biotherapeutics, providing insights into the intricate details of aggregate formation and charge variants.
SIZE EXCLUSION CHROMATOGRAPHY MASS SPECTROMETRY (SEC-MS)
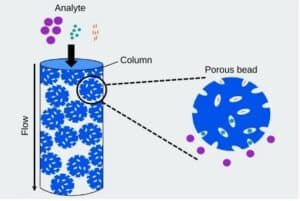
SEC-MS represents a powerful analytical technique that combines the separation capabilities of size exclusion chromatography with the mass analysis capabilities of mass spectrometry. In this method, biomolecules, such as proteins or peptides, are first separated based on their size in a chromatographic column with porous beads. As molecules elute from the column, they are directed into the mass spectrometer for further characterization.
Fig: A schematic diagram of the separation principle of Size Exclusion Chromatography.
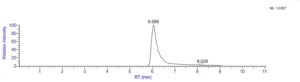
Fig: A representative chromatogram of the analytes’ elution profile in SEC-MS.
- Size Determination:
a. Protein Size: SEC separates biomolecules based on their size and coupling it with MS provides accurate mass determination. This is crucial for confirming the expected size of proteins, especially in the case of biotherapeutics where the correct size is essential for proper function.
b. Aggregation Analysis: SEC-MS can be used to detect and characterize protein aggregates, which is a critical parameter in the development of biotherapeutics to ensure product safety and efficacy. - Purity Assessment:
a. Impurity Detection: SEC-MS can help identify and quantify impurities in biotherapeutic samples. This is important for ensuring product quality and meeting regulatory requirements. - Quality Control in Manufacturing:
a. Batch-to-Batch Consistency: SEC-MS is used in the quality control of biotherapeutic manufacturing processes to ensure consistency in product quality between different batches. - Biosimilar Development:
a. Comparability Studies: When developing biosimilars, SEC-MS can be employed to conduct comparability studies between the biosimilar and the reference product, ensuring that they are structurally similar. - Stability Studies:
a. Storage and Formulation Stability: SEC-MS can be used to assess the stability of biotherapeutics under different storage conditions and in various formulations, providing information on potential degradation pathways.
Fig: SEC-MS analysis of an antibody drug conjugate (ADC).
Fig: Deconvoluted spectrum of the ADC.
ION EXCHANGE CHROMATOGRAPHY MASS SPECTROMETRY (IEX-MS)
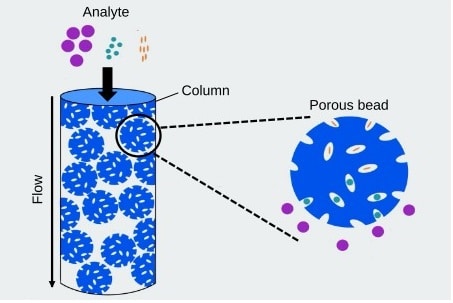
IEX-MS is an analytical approach extensively applied in the analysis of biotherapeutics. This technique integrates the high-resolution separation capabilities of ion exchange chromatography with the precise mass analysis provided by mass spectrometry. In the context of biotherapeutics, IEX-MS is particularly useful for characterizing charged species, such as proteins and peptides. By exploiting the differences in the net charge of biomolecules, IEX separates them based on their interactions with charged stationary phases. When coupled with mass spectrometry, this method enables the identification, quantification, and characterization of various charge variants, post-translational modifications, and impurities within biotherapeutic samples.
Fig: A schematic diagram of the separation principle of Cation Exchange Chromatography (CEX), a type of IEX.
IEX-MS plays a critical role in ensuring the quality and consistency of biopharmaceutical products by providing detailed insights into the charge heterogeneity and structural attributes of therapeutic proteins, aiding researchers and manufacturers in optimizing production processes and meeting stringent regulatory requirements.
- Charge Variant Analysis:
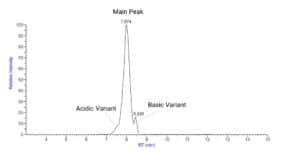
a. Identification of Charge Variants: IEX-MS is used to separate and identify different charge variants of biotherapeutics. This is crucial because changes in charge variants may affect the bioactivity, stability, and pharmacokinetics of the therapeutic protein. - Purity Assessment:
a. Impurity Detection: IEX-MS helps in the detection and characterization of impurities in biotherapeutic samples. This is important for assessing the purity of the final product and meeting regulatory requirements. - Post-translational Modification (PTM) Analysis:
a. Phosphorylation, Acetylation, etc.: IEX-MS can be applied to study and quantify various post-translational modifications, providing insights into the structural heterogeneity of biotherapeutics. - Biosimilar Development:
a. Comparability Studies: IEX-MS is utilized in comparability studies between a biosimilar and the reference product. It helps ensure that the biosimilar has similar charge properties to the reference product. - Quality Control in Manufacturing:
a. Batch-to-Batch Consistency: IEX-MS is used for quality control purposes in the manufacturing of biotherapeutics to ensure consistency in charge properties between different batches. - Stability Studies:
a. Forced Degradation Studies: IEX-MS is employed in stability studies to assess the impact of stress conditions on the charge variants and overall stability of biotherapeutics.
Fig: CEX-MS analysis of the NISTmAb.
Fig: Deconvoluted spectrum of the NISTmAb.
In essence, SEC-MS, and IEX-MS stand at the forefront, driving the biologics research and development, offering comprehensive characterization, quality control capabilities, and insights into stability and structural attributes critical for ensuring the safety, efficacy, and regulatory compliance of biopharmaceutical products.