The Past, Present and Future of ADCs
ADCs, short for antibody-drug conjugates, consist of three essential components: a monoclonal antibody (mAb), a linker molecule, and a small molecule drug. In simple terms, an ADC is created by joining an antibody (mAb) with a drug through a linker or spacer. The linker is a chemical bridge that attaches the drug to the antibody, usually at specific sites like lysine or cysteine residues. The drug connected to the antibody is commonly referred to as the “payload.”
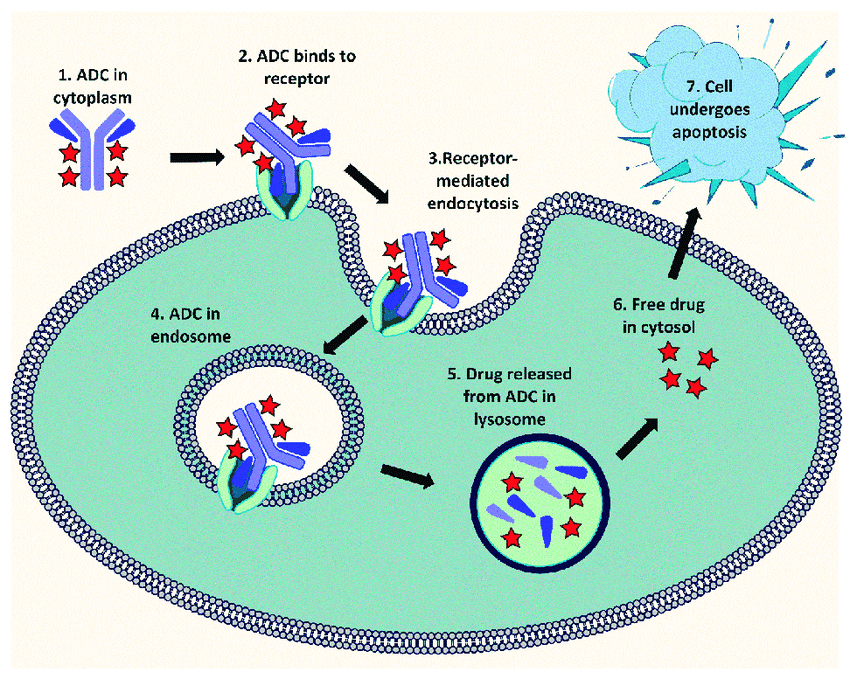
For instance, in the realm of oncology therapeutics, the antibody is designed to target antigens that are abundantly present on the surface of cancer cells. The primary objective is to deliver the cytotoxic drug selectively to these cancer cells while sparing non-target cells—a challenge that plagues many traditional chemotherapy approaches. This precision in drug delivery makes ADCs exceptionally potent, potentially inducing apoptosis18, or programmed cell death, specifically in the cells we aim to eliminate. It’s a compelling approach to treating various diseases, particularly cancer.
Figure 1. A diagram illustrating the mechanism of action of Antibody-Drug Conjugate (ADC) in a cancer cell (Arpita Datta et. Al)
With a solid foundation in the basics of ADCs, let’s now embark on a journey through their fascinating history. We’ll explore how these innovative therapies have evolved and the milestones that have shaped their development. From humble beginnings to the cutting-edge treatments of today, the history of ADCs is a testament to scientific innovation and the relentless pursuit of more effective, targeted therapies.
It all began with a visionary concept dating back to 1907 when Nobel laureate Paul Ehrlich introduced the idea of “zauberkugel” or the magic bullet—a compound capable of selectively homing in on pathogenic cell structures while leaving healthy cells unscathed23. Ehrlich also coined the term “chemotherapy,” highlighting the use of chemicals to combat disease. Despite these early visionary ideas, progress was impeded by the toxicity of the toxins and the lack of effective targeting2.
Nonetheless, research in the field persisted. In the 1960s, the first documented in vitro studies exploring ADCs took place. Researchers used antibodies conjugated to radioactive isotopes or cytotoxic drugs to target tumor cells11. However, these early ADCs had several limitations, such as low drug-to-antibody ratio, unstable linkers, and poor pharmacokinetics21. The breakthrough came in the 1970s, when monoclonal antibodies were developed by Kohler and Milstein11. These mAbs offered a higher degree of specificity and diversity of targets than the previous polyclonal antibodies. They also opened up new possibilities for ADC development, as they could be engineered to recognize various antigens on cancer cells21,11.
Before the advent of monoclonal antibodies, early ADCs relied on polyclonal antibody conjugates, offering less target specificity and potentially higher toxicity. This shifted in 1983 with the first human trial of an ADC using a mAb was conducted, with an anti-carcinoembryonic antigen antibody-vindesine conjugate21. This ADC was deemed to be both safe and effective in the eight patients with various advanced metastatic carcinomas21. Another significant milestone occurred in 1986 with the FDA approval of the first monoclonal antibody, muromonab-CD3, designed to reduce acute rejection in organ transplant patients4. While this antibody didn’t incorporate a linker and drug, its approval marked a crucial turning point in the realm of antibody-based therapeutics.
Fast forward to the year 2000, and a groundbreaking moment unfolded with the approval of the first ADC, gemtuzumab (Mylotarg)2. This ADC featured a CD33 monoclonal antibody conjugated to a calicheamicin drug, initially targeting blood-borne cancers.
Then, in 2013, another advancement occurred when Trastuzumab emtansine (Kadcyla) gained approval for HER2-positive breast cancer. This marked a pivotal moment in the history of ADCs, showcasing their potential not just in blood-based cancers but also in addressing solid tumors.
The emergence of Trastuzumab emtansine ignited a wave of excitement and substantial investments in ADC research. However, it’s important to recognize that not all ADCs have shared the same success story. Some have faced significant hurdles during clinical development and commercialization, experiencing safety or efficacy concerns. For instance, the first approved ADC, Mylotarg, was withdrawn after subsequent confirmatory trials failed to verify clinical benefit and demonstrated safety concerns, including a high number of early deaths. It was later reintroduced into the market27.
Additional examples include:
- Inotuzumab ozogamicin (Besponsa): This ADC, designed to target CD22—a marker of B-cell malignancies—and deliver a calicheamicin payload, initially received FDA approval in 2017 for the treatment of relapsed or refractory acute lymphoblastic leukemia (ALL). However, its journey took a turn in 2019 after failing to demonstrate a survival benefit over standard chemotherapy in a Phase III DLBCL Additionally, it carries a boxed warning for hepatotoxicity, including veno-occlusive disease (VOD), which can be fatal14.
- Vadastuximab talirine (SGN-CD33A): Targeting CD33, a marker of acute myeloid leukemia (AML), and delivering a PBD payload, this ADC faced a significant setback. It was discontinued in 2016 after a Phase III trial was halted due to a higher rate of deaths, including fatal infections, among patients treated with the ADC plus chemotherapy compared to chemotherapy alone. It also exhibited a higher incidence of VOD than anticipated24,17.
- Rovalpituzumab tesirine (Rova-T): This ADC, targeting DLL3—a marker of small cell lung cancer (SCLC)—and delivering a pyrrolobenzodiazepine (PBD) payload, faced challenges of its own. It was discontinued in 2019 after failing to demonstrate a survival benefit over standard chemotherapy in two Phase III trials for second-line and third-line SCLC. Additionally, it had a high rate of adverse events, including thrombocytopenia, photosensitivity, and pleural effusion19,1.
These examples illustrate some of the challenges that ADCs face in terms of safety and efficacy, such as toxicity, immunogenicity, antigen expression and internalization, drug resistance, and patient selection. To overcome some of these limitations of the first-generation ADCs, researchers began exploring new ways to optimize the design and performance of ADCs. Some of the recent trends and innovations in ADC development include:
- Novel Payloads: Beyond the tried-and-true tubulin inhibitors and DNA cross-linkers, ADC research now explores a new frontier of cytotoxic agents. These include DNA alkylators like duocarmycin, RNA polymerase inhibitors such as anthracyclines, DNA minor groove binders like distamycin, proteasome inhibitors such as bortezomib, kinase inhibitors like auristatin F, and immunomodulators like IL-26. These novel payloads bring diverse mechanisms of action, potencies, stability, and toxicity profiles, expanding the therapeutic potential of ADCs34.
- Novel Linkers: The linker within an ADC is pivotal, determining payload stability, release, and pharmacokinetics. Researchers are developing new types of linkers to enhance the control and specificity of payload delivery. These innovations encompass cleavable linkers sensitive to pH, enzymes, or redox conditions; non-cleavable linkers resistant to degradation; and hybrid linkers that seamlessly combine both features9,3. Some linkers may even have additional functions, such as enhancing solubility, reducing aggregation, or modulating immunogenicity7.
- Novel Conjugation Strategies: The way in which the payload attaches to the antibody, known as the conjugation strategy, is also evolving. Traditional methods involve random conjugation to lysine or cysteine residues on the antibody, often resulting in heterogeneous ADCs with varying drug-to-antibody ratios (DARs) and biological properties33. New methods aim to achieve site-specific conjugation to engineered or native sites on the antibody. These sites may include glycan residues, unnatural amino acids, or interchain disulfide bonds. Such innovations produce more homogeneous ADCs with well-defined DARs, ultimately improving stability and efficacy15. Additional conjugation strategies include dual warhead conjugation, delivering two different payloads to the same target; bispecific conjugation, targeting two different antigens on the same or different cells; and multivalent conjugation, enhancing binding avidity and potency9.
Currently, there are 11 FDA-approved ADCs for both solid and hematologic malignancies that are on the market25, and the field is still thriving, exemplified by the approval of eight ADCs between 2019 and 2022 alone5. Moreover, 249 clinical trials evaluating ADCs were initiated in 2022, reflecting the continued growing attention in the field5.
Some of the most notable ADCs that have been approved are:
- Brentuximab vedotin (Adcetris): This ADC consists of an anti-CD30 mAb conjugated to monomethyl auristatin E (MMAE), a microtubule-disrupting agent. It was approved by the FDA in 2011 for relapsed or refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. It has also shown promising results in other CD30-positive malignancies such as cutaneous T-cell lymphoma and diffuse large B-cell lymphoma26.
- Polatuzumab vedotin (Polivy): This ADC consists of an anti-CD79b mAb conjugated to MMAE. It was approved by the FDA in 2019 for relapsed or refractory diffuse large B-cell lymphoma in combination with bendamustine and rituximab. It has also demonstrated efficacy in other B-cell malignancies such as follicular lymphoma and mantle cell lymphoma28.
- Sacituzumab govitecan (Trodelvy): This ADC consists of an anti-TROP2 mAb conjugated to SN-38, the active metabolite of irinotecan, a topoisomerase I inhibitor. It was approved by the FDA in 2020 for metastatic triple-negative breast cancer. It has also shown activity in other solid tumors such as urothelial carcinoma, non-small cell lung cancer, and colorectal cancer30.
- Enfortumab vedotin (Padcev): This ADC consists of an anti-Nectin-4 mAb conjugated to MMAE. It was approved by the FDA in 2019 for locally advanced or metastatic urothelial carcinoma that has progressed after platinum-based chemotherapy and a PD-1/PD-L1 inhibitor. It has also shown potential in other Nectin-4-positive tumors such as breast cancer and head and neck cancer31.
- Loncastuximab tesirine (Zynlonta): This ADC consists of an anti-CD19 mAb conjugated to pyrrolobenzodiazepine (PBD) dimer, a DNA cross-linking agent. It was approved by the FDA in 2021 for relapsed or refractory diffuse large B-cell lymphoma. It has also demonstrated efficacy in other CD19-positive malignancies such as mantle cell lymphoma and chronic lymphocytic leukemia29.
However, this surge in recent interest also presents its own set of challenges. There’s a notable overlap in clinical programs, with over 20% focusing on HER2 and TROP2 malignancies due to their suitability as proof of concept, yielding marginal gains for patients5.
Despite these challenges, the field continues to evolve, with newer research into linkers and payloads driving a noteworthy shift in focus. For example, before 2022, 51% of all payloads utilized tubulin inhibitors, whereas only 25% of the new ADCs disclosed in 2022 employed tubulin inhibitors5.
Even more exciting, the ADC landscape is starting to go beyond oncology, as research now explores novel ADC therapeutics for diseases such as severe autoimmune disorders and neurodegenerative disorders, such as Alzheimer’s32,12. These developments underscore the ever-evolving and diverse potential of ADCs, demonstrating their adaptability across a wide range of medical challenges.
As we look ahead, the prospects for ADCs are indeed bright, but substantial work remains. Much of the current and future research predominantly focuses on a few key areas:
First, optimizing dosing and administration schedules is essential. Tailoring the ideal regimen for each ADC is complex, as it depends on factors like pharmacokinetics, pharmacodynamics, toxicity, efficacy, and patient characteristics. There is no one-size-fits-all approach, necessitating extensive studies to evaluate different dosing regimens (e.g., dose escalation, dose reduction, dose fractionation) and administration routes (e.g., intravenous, subcutaneous) to enhance both safety and efficacy8.
Additionally, exploring the combination of ADCs with other cancer treatment modalities, such as immunotherapy (e.g., checkpoint inhibitors), radiotherapy (e.g., radionuclide conjugates), or targeted therapy (e.g., tyrosine kinase inhibitors), holds promise. These combinations may boost anti-tumor activity by influencing the tumor microenvironment, increasing antigen expression or internalization, overcoming drug resistance, or inducing immunogenic cell death. However, they may also heighten the risk of toxicity or adverse events. Thus, further research is needed to pinpoint the optimal combination partners and strategies for ADCs16,22,20.
Lastly, the development of predictive biomarkers and companion diagnostics is a critical challenge. These tools, which assist in identifying patients likely to respond to a specific treatment, hold the potential to personalize ADC therapy by matching the right patients with the right ADCs at the right doses. Nonetheless, the complexity and heterogeneity of ADCs and tumors make developing reliable biomarkers and diagnostics a demanding task, necessitating further research for discovery and validation10,13.
Works Cited
- “AbbVie Discontinues Rovalpituzumab Tesirine (Rova-T) Research and Development Program | AbbVie News Center.” abbvie.com, 29 Aug. 2019, news.abbvie.com/news/press-releases/abbvie-discontinues-rovalpituzumab-tesirine-rova-t-research-and-development-program.htm.
- “Antibody-Drug Conjugates (ADCs) – Approvals & Clinical Trails | Biopharma PEG.” biochempeg.com, 12 Aug. 2021, www.biochempeg.com/article/208.html.
- Bargh, Jonathan D., et al. “A Dual-Enzyme Cleavable Linker for Antibody–Drug Conjugates.” Chemical Communications, vol. 57, no. 28, 2021, pp. 3457–60, https://doi.org/10.1039/d1cc00957e. Accessed 20 Jan. 2022.
- Barnes, Katharine. “The First Monoclonal Antibody Therapy.” Nature Research, Dec. 2018, https://doi.org/10.1038/d42859-018-00024-6.
- Barnscher, Stuart. “The Clinical Landscape of ADCs in 2023: Diverse Technologies, Narrow Target.” clinicalleader.com, 16 Apr. 2023, www.clinicalleader.com/doc/the-clinical-landscape-of-adcs-in-diverse-technologies-narrow-target-0001.
- Camper, Nicolas. “Antibody-Drug Conjugates Payloads: Then, Now and Next.” Drug Target Review, 4 Oct. 2023, www.drugtargetreview.com/article/111783/antibody-drug-conjugates-payloads-then-now-and-next/. Accessed 9 Nov. 2023.
- Chuprakov, Stepan, et al. Tandem-Cleavage Linkers Improve the in Vivo Stability and Tolerability of Antibody-Drug Conjugates. Jan. 2021, https://doi.org/10.1101/2021.01.19.427340. Accessed 9 Nov. 2023.
- Clinical Pharmacology Considerations for Antibody-Drug Conjugates Guidance for Industry DRAFT GUIDANCE Clinical Pharmacology. 2022, www.fda.gov/media/155997/download.
- Criscitiello, Carmen, et al. “Antibody–Drug Conjugates in Solid Tumors: A Look into Novel Targets.” Journal of Hematology & Oncology, vol. 14, no. 1, Jan. 2021, https://doi.org/10.1186/s13045-021-01035-z.
- Dornan, David, and Jeff Settleman. “Predictive Biomarkers for Antibody–Drug Conjugates.” Springer EBooks, Nov. 2012, pp. 77–90, https://doi.org/10.1007/978-1-4614-5456-4_5. Accessed 9 Nov. 2023.
- Eder, Michael. “Development & History of Antibody Drug Conjugates (ADCs).” susupport.com, 14 Apr. 2023, www.susupport.com/knowledge/bioconjugates/history-development-adcs. Accessed 9 Nov. 2023.
- “Exploring the Latest Trends in Antibody Therapeutics | Bio-Rad.” bio-Rad.com, www.bio-rad.com/en-us/applications-technologies/exploring-latest-trends-antibody-therapeutics?ID=a75a5fba-560b-295f-b90b-6582dddb6e7e. Accessed 9 Nov. 2023.
- Huber, Cynthia, et al. “Classification of Companion Diagnostics: A New Framework for Biomarker- Driven Patient Selection.” Therapeutic Innovation & Regulatory Science, vol. 56, no. 2, Nov. 2021, pp. 244–54, https://doi.org/10.1007/s43441-021-00352-2.
- Inman, Silas. “Inotuzumab Ozogamicin Receives FDA Approval for ALL.” Targeted Oncology, 17 Aug. 2017, www.targetedonc.com/view/inotuzumab-ozogamicin-receives-fda-approval-for-all. Accessed 9 Nov. 2023.
- J. Walsh, Stephen, et al. “Site-Selective Modification Strategies in Antibody–Drug Conjugates.” Chemical Society Reviews, vol. 50, no. 2, 2021, pp. 1305–53, https://doi.org/10.1039/D0CS00310G. Accessed 31 Mar. 2021.
- Jacobsen, Sonja. “What You Should Know about Antibody-Drug Conjugates for Cancer.” GoodRx, GoodRx, 29 Aug. 2022, www.goodrx.com/conditions/cancer/adc-cancer. Accessed 9 Nov. 2023.
- July 10, The ASCO Post Staff, and 2017. “Phase III CASCADE Trial of Vadastuximab Talirine in Front-Line AML Discontinued – the ASCO Post.” Ascopost.com, 10 July 2017, ascopost.com/issues/july-10-2017/phase-iii-cascade-trial-of-vadastuximab-talirine-in-front-line-aml-discontinued/. Accessed 9 Nov. 2023.
- Khongorzul, Puregmaa, et al. “Antibody–Drug Conjugates: A bio Review.” Molecular Cancer Research, vol. 18, no. 1, Jan. 2020, pp. 3–19, https://doi.org/10.1158/1541-7786.MCR-19-0582.
- Mullard. “Cancer Stem Cell Candidate Rova-T Discontinued.” Nature Reviews Drug Discovery, vol. 18, no. 11, Oct. 2019, pp. 814–14, https://doi.org/10.1038/d41573-019-00176-8. Accessed 26 Jan. 2021.
- Nagayama, Aiko, et al. “Antibody–Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments.” Targeted Oncology, vol. 12, no. 6, Nov. 2017, pp. 719–39, https://doi.org/10.1007/s11523-017-0535-0. Accessed 26 June 2019.
- Nawrat, Allie. “Charting the Choppy History of ‘Magic Bullet’ Antibody-Drug Conjugates.” Www.pharmaceutical-Technology.com, 10 Feb. 2021, www.pharmaceutical-technology.com/features/antibody-drug-conjugates-timeline/.
- Nicolò, Eleonora, et al. “Combining Antibody-Drug Conjugates with Immunotherapy in Solid Tumors: Current Landscape and Future Perspectives.” Cancer Treatment Reviews, vol. 106, May 2022, p. 102395, https://doi.org/10.1016/j.ctrv.2022.102395. Accessed 8 Sept. 2022.
- “Paul Ehrlich Centenary Award | BioIron – International Society for the Study of Iron in Biology and Medicine.” Bioiron.org, 2019, bioiron.org/about-us/awards/paul-ehrlich-centenary-award.aspx#:~:text=He%20introduced%20the%20notion%20of%20zauberkugel%20or%20magic. Accessed 9 Nov. 2023.
- “Phase III Trials of Vadastuximab Talirine Discontinued amid Safety Concerns.” Ashpublications.org, Dec. 2021, ashpublications.org/ashclinicalnews/news/3240/Phase-III-Trials-of-Vadastuximab-Talirine. Accessed 9 Nov. 2023.
- Pooja Gogia, et al. “Antibody–Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence.” Cancers, vol. 15, no. 15, Multidisciplinary Digital Publishing Institute, July 2023, pp. 3886–86, https://doi.org/10.3390/cancers15153886. Accessed 30 Oct. 2023.
- Research, Center for Drug Evaluation and. “Brentuximab Vedotin.” FDA, Feb. 2019, www.fda.gov/drugs/resources-information-approved-drugs/brentuximab-vedotin.
- “FDA Approves Gemtuzumab Ozogamicin for CD33-Positive AML.” FDA, Feb. 2019, www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-gemtuzumab-ozogamicin-cd33-positive-aml.
- “FDA Approves Polatuzumab Vedotin-Piiq for Diffuse Large B-Cell Lymphoma.” FDA, Dec. 2019, www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-polatuzumab-vedotin-piiq-diffuse-large-b-cell-lymphoma.
- “FDA Grants Accelerated Approval to Loncastuximab Tesirine-Lpyl for Large B-Cell Lymphoma.” FDA, June 2021, www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-loncastuximab-tesirine-lpyl-large-b-cell-lymphoma.
- “FDA Grants Accelerated Approval to Sacituzumab Govitecan for Advanced Urothelial Cancer.” FDA, Apr. 2021, www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sacituzumab-govitecan-advanced-urothelial-cancer.
- “FDA Grants Regular Approval to Enfortumab Vedotin-Ejfv for Locally Advanced or Metastatic Urothelial Cancer.” FDA, July 2021, www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer.
- Taylor, Nick. “The Accelerating Rise of Bispecific Antibodies outside of Oncology.” Biopharma Dealmakers, Sept. 2018, https://doi.org/10.1038/d43747-020-00566-7. Accessed 9 Nov. 2023.
- Tsuchikama, Kyoji, and Zhiqiang An. “Antibody-Drug Conjugates: Recent Advances in Conjugation and Linker Chemistries.” Protein & Cell, vol. 9, no. 1, Oct. 2016, pp. 33–46, https://doi.org/10.1007/s13238-016-0323-0. Accessed 8 Feb. 2019.
- “Vincerx—Next-Generation Antibody–Drug Conjugates with Enhanced Therapeutic Windows.” Www.nature.com, Apr. 2021, www.nature.com/articles/d43747-021-00011-3. Accessed 9 Nov. 2023.