GLP Compliant CRO Services
GLP, or “Good Laboratory Practice,” refers to a set of federal regulations established by the U.S. Food and Drug Administration (FDA) to ensure the quality and integrity of non-clinical laboratory studies. These regulations are primarily outlined in 21 CFR Part 58, which governs non-clinical laboratory studies, and 21 CFR Part 11, which addresses electronic records and signatures. Compliance with GLP is essential for laboratories conducting studies that support applications for FDA approval of pharmaceuticals, biologics, and chemicals. Contract Research Organizations (CROs) are considered GLP-compliant when they have implemented comprehensive quality management systems that ensure data validity, consistency, and traceability.
At Emery Pharma, our quality system is designed to uphold the highest GLP standards. We provide meticulous execution, documentation, review, and archiving of data related to non-clinical and environmental safety studies. This ensures that the results we generate are scientifically sound, reproducible, and fully aligned with regulatory expectations, supporting our clients’ regulatory submissions with confidence.
All regulated testing as required for product or material release of pharmaceuticals, excipients, reference standards, etc. are performed at Emery Pharma in compliance to cGMP (Current Good Manufacturing Practices) per 21 CFR Part 210 and 211.
Emery Pharma follows approved standard operating procedures (SOPs), test methods, and protocols to ensure all aspect of our laboratory operations are followed per cGMP. All instruments used for cGMP testing are appropriately qualified and regularly verified for acceptable operation and performance.
These standards are maintained by the audits performed by internal audits, regulatory inspections performed by FDA, as well as external (Client/Sponsor) audits.
Note: The Client/Sponsor bears the responsibility for assessing all testing and validation/verification requirements for their product. Further, the Client/Sponsor is responsible for determining the suitability and all regulatory acceptance of their product and/or testing program.
Emery Pharma’s Scientists, Quality Assurance (QA), and Regulatory experts are happy to assist in determining such requirements and conformance to cGMP.
Emery Pharma’s Quality System
Overview
Emery Pharma’s Quality System ensures compliance with the laws and regulations pertaining to the quality, safety, and performance requirements for the company’s testing services. A dedicated Quality Assurance department at Emery Pharma implements all planned and systematic activities within the quality system.
An established Quality Assurance department regularly reviews this Quality Policy and trends the quality objectives set forth, aiming for continuous improvement of the quality system to assure that we can provide the highest standard of service.
To commit ourselves to the quality of our services, we will comply with the laws and regulations set forth in FDA’s GLP (21 CFR Part 58) and cGMP (21 CFR Part 210 and 211) guidelines.
Quality Objectives
- Continue to maintain, implement, develop, and seek improvements in the effectiveness of the quality system in compliance with FDA GLP and cGMP as required.
- Ensure compliance with all data integrity requirements, such as 21 CFR Part 11.
- Ensure no one at Emery Pharma has any conflict of interest with regulated projects.
- Ensure all analysts are familiar with the quality documentation and implement the policies and procedures in their work.
- Ensure all equipment and instruments used are properly maintained and calibrated, traceable to recognized standards.
- Use internal audits and other procedures to ensure all policies and procedures are being followed, as well as ensure that the quality system continues to comply with national and international requirements.
- Ensure any problems that occur are investigated, root causes are established, and effective corrective and/or preventive actions (CAPAs) are taken to prevent a recurrence.
- Meet Client requirements and expectations of sample turnaround time and service reliability.
- Seek feedback from Clients to aid in continuous improvement.
- Follow a Customer Complaint procedure to resolve Client issues.
Quality Assurance (QA)
Emery Pharma’s Quality Assurance (QA) Department assures that protocols, methods, practices, records, and controls all conform with applicable regulatory standards and internal standards. We support GLP and cGMP studies across all stages of pharmaceutical discovery and development (see below).
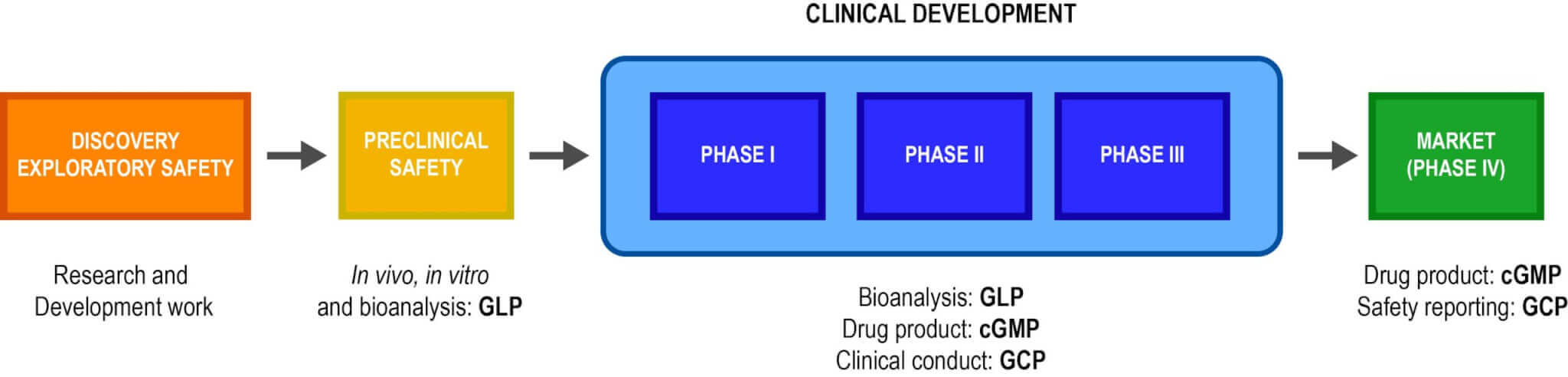
Our Quality System has been designed to achieve compliance with FDA GLPs (21 CFR part 58) and cGMPs (21 CFR parts 210 and 211). Additionally, we take guidance from the requirements of non-clinical testing laboratories in ISO 17025.
Emery Pharma specializes in developing custom methods for our Clients, as well as supporting new drug modalities (e.g., botanical drugs). We strongly recommend consulting our QA, Scientists, and Regulatory experts to discuss if your project is applicable to these standards.
Our Quality Policy Statement
Quality is the foundation of everything we do at Emery Pharma. We commit ourselves to delivering scientifically rigorous, accurate, and reliable testing services that meet or exceed our client’s expectations and deadlines while complying with applicable national and international standards. To uphold this commitment, we continually maintain and improve a robust Quality Management System (QMS) that supports data integrity, traceability, and scientific excellence.
