Drug Lipophilicity and Absorption: The Continuous Challenge in Drug Discovery
Oral delivery is the well-known administrative drug discovery path accepted by both patients and manufacturers. According to the recent report published in pharmaceutical technology, achieving optimal physicochemical properties of biomolecules, including size and degree of surface polarity is an ongoing challenge toward the oral delivery of drugs. In the case of larger biomolecules with a higher degree of surface polarity, their absorption across the gastrointestinal (GI) tract is limited due to their insufficient degree of drug lipophilicity. [1]
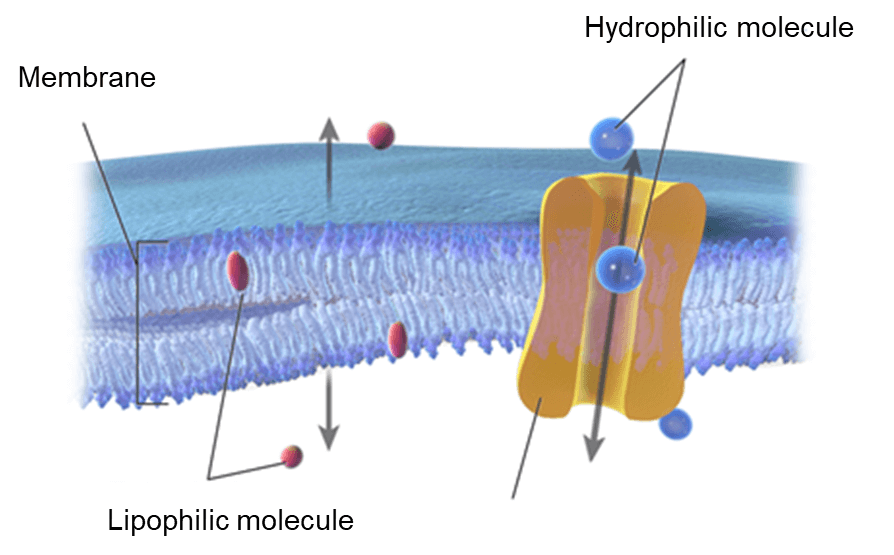
Drug discovery is a key step in the development of a drug candidate. It commonly involves a wide range of scientific disciplines, including Microbiology, Chemistry, and Pharmacology. The process is initiated by the testing of many new potential molecules against various biochemical targets. Afterwards, the compounds that exhibit significant activities may be considered as drug candidates for further development. In addition to the activity of the drug candidates, absorption and distribution of the drug candidates in the human body are considered critical. The most common transport route of a drug is based on the drug absorption through membranes via passive diffusion. It is driven by the tendency of the drug to absorb into a new environment with no need for the input of cellular energy. Therefore, the drug candidates must be lipophilic enough to penetrate to lipid core of membranes but not too lipophilic that they remain there. [2]
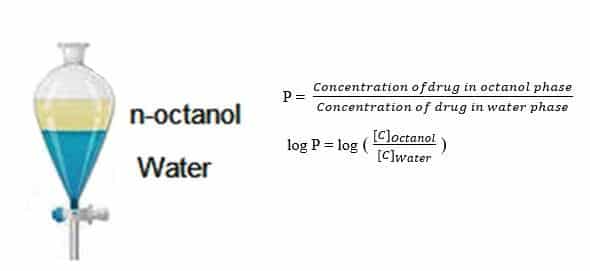
Lipophilicity is defined as the affinity of a drug for a lipid environment. It has become a critical parameter in the Pharmaceutical industry, which indicates the relationship of a drug with their biological, pharmacokinetic, and metabolic properties. Lipophilicity can be measured by the distribution of a drug between the organic phase, which is generally n-octanol pre-saturated with water, and the aqueous phase, which is generally water pre-saturated with n-octanol. The partition coefficient (P) is referred to as the ratio of the equilibrium concentrations (Ci) of a dissolved compound in a two-phase system consisting of n-octanol and water.
The partition coefficient (P) is usually given in the form of its logarithm to base 10 (log P). The log P value represents the partition constant of the compound in neutral form. The distribution coefficient (log D) represents the partition coefficient of the compound in both neutral and ionized form at any pH. The most relevant descriptor accepted by most scientists is log D7.4. The log D7.4 of a compound represents its distribution coefficient at pH 7.4, which resembles real biological and physiological partitions. Log P and log D can be experimentally determined using various methods, including shake-flask method, chromatographic, and potentiometric methods.
The shake-flask method is known as reference method compared to the other ones due to its simplicity and clear relationship to the partitioning phenomenon. As shown in the figure below, in this method, an aqueous solution of a drug is mixed in a flask with an organic solvent (typically n-octanol saturated with water). Then, the flask is shaken to equilibrate the sample between the two phases, and the phases are then separated. Afterwards, the concentration of the analyte is measured in both phases. Ultraviolet and visible (UV-Vis) spectroscopy and high-performance liquid chromatography (HPLC) techniques are most widely used to measure the concentration of the drug in each phase.
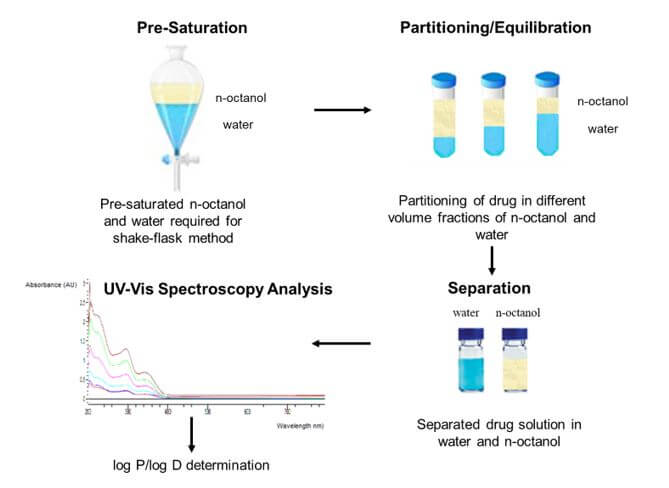
At Emery Pharma, our team of bioanalytical scientists supports drug discovery companies in determination of log P and log D values of drug candidates by using the Organization for Economic Co-operation and Development (OECD) standard guideline of the shake-flask method followed by UV-Vis and HPLC analysis.[3] It is worth mentioning that the standard shake-flask method requires the appropriate selection of volume fraction of phases, drug concentration in the aqueous phase, and relative ionic strength of buffer system at certain pH value to achieve the accurate analysis of the drug in both phases. According to our recent studies at Emery Pharma, it appears that the nature of the counter ion in a form of a drug salt can significantly influence the log P/log D value of the drug, which needs to be corrected for the accurate prediction of log P/log D value of the drug by software. If you need to evaluate the lipophilicity of your drug candidates, please contact us at info@emerypharma.com.
About the Author
Al (Ali) Najafi holds a Master of Science in Analytical Chemistry and he is currently working as a Research Associate at Emery Pharma.
References
[1] Challener, Cynthia A. “Oral Delivery of Biologic APIs: The Challenge Continues.” PharmTech Home, 10 Apr. 2017.
[2] Waterbeemd, Han Van de, and Bernard Testa. Drug Bioavailability Estimation of Solubility, Permeability, Absorption and Bioavailability. Wiley-VCH, 2009.
[3] OECD, Paris, 1981, Test Guideline 107, Decision of the Council C (81) 30 final.
[4] “Life in Research” Cartoon # 500, Vadlo Life science search engine online.
[5] Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. Passive Transport, Wikipedia.
