Characterization of Biologic Drugs: An Overview
Intact Mass and Subunit Fragment Analyses
The LC-MS analyses of the intact and subunit biologic molecules are simple analyses which characterize detailed modifications of complex molecules. This information-rich study is useful throughout the product development cycle, from research to commercialization, including GMP product release, comparability, and characterization studies. Indeed, the consistency of drug production is supported by the consistency of intact mass results throughout the project lifetime. The intact LC-MS analyses may also be utilized as an ID test in commercial release testing. These analyses are applicable to a wide variety of protein/peptide-based biopharmaceuticals (mAbs, ADCs, etc.).
Intact mass analyses are performed via LC-MS with minimal sample preparation and straightforward data interpretation. Generally, the drug substance or the drug product is diluted and injected in an LC-MS method. The results of the intact mass analysis can confirm the expected mass of the protein (ID), identify the major glycosylations, and any C- and/or N-terminal modifications.
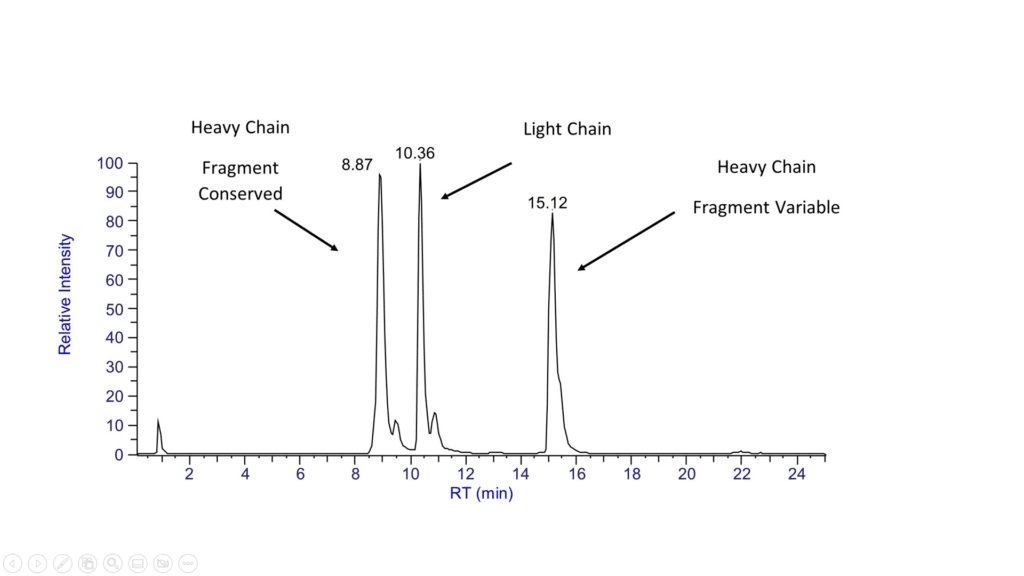
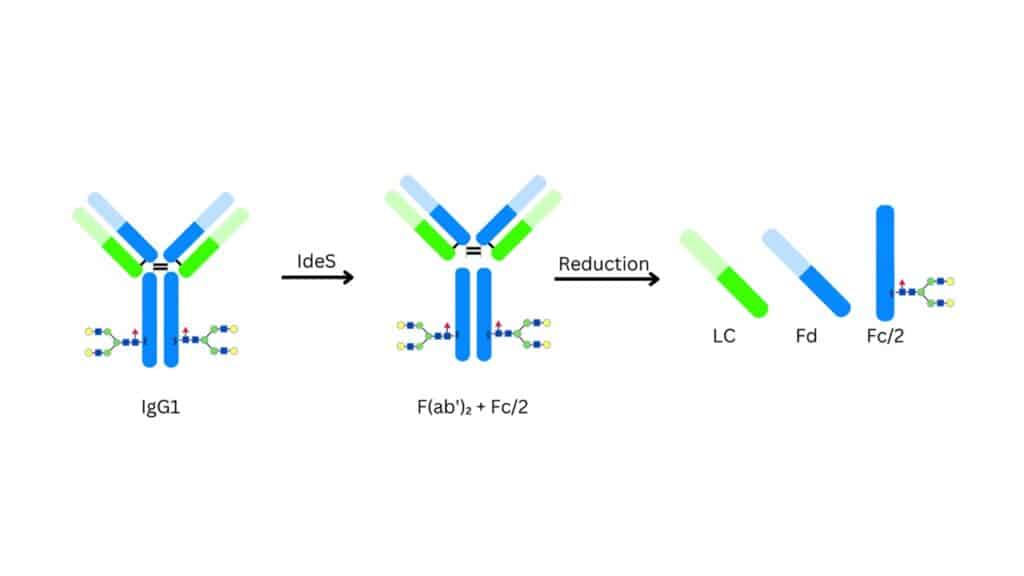
Subunit mass analyses require additional sample preparation steps. Subunits can be prepared by the reduction of inter-chain disulfide bonds, by using enzymes to cleave a specific bond in the hinge region of a mAb, by enzymatically releasing the N-glycosylation, or any combination of these. The results of the fragment analyses can additionally identify modifications such as oxidations and glycations, and can assign these modifications to a particular fragment. The analysis of the de-glycosylated protein offers insight into the core biopharmaceutical structure and is often mandated by internal quality protocols.
At Emery Pharma, intact and fragment mass analyses are performed on a Thermo Scientific™ Exploris 240 UPLC-MS system with the Biopharma option that offer unprecedented mass range for intact mass analyses (m/z up to 8000).
Peptide Mapping
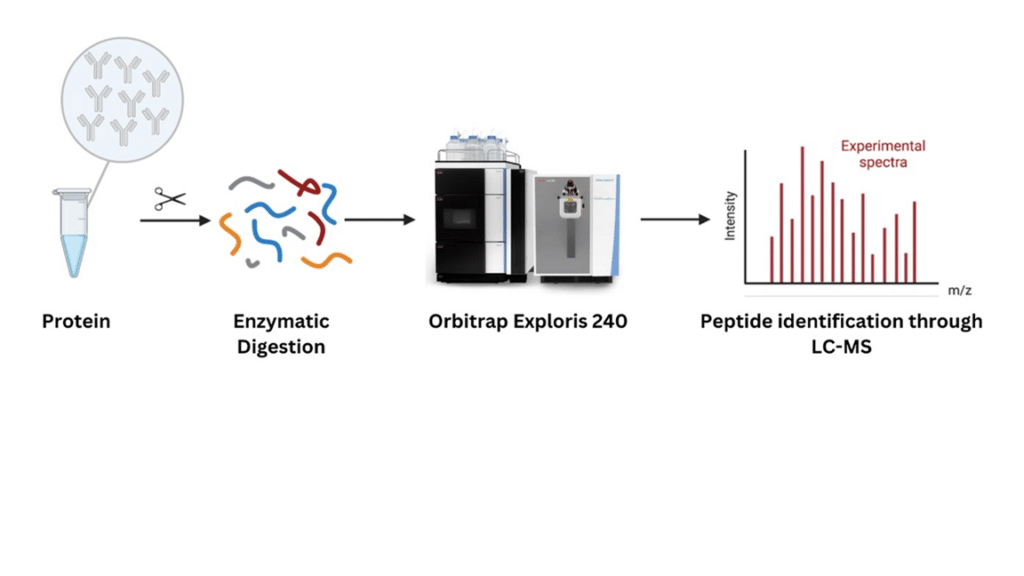
Peptide mapping is the digest of a protein by an enzyme, typically trypsin, into several smaller peptides which are then separated by chromatography and analyzed by either a mass spectrometer (LC-MS) or UV (LC-UV) detector. Additional proteolytic enzymes (e.g., Lys-C, Glu-C, chymotrypsin) may be considered to achieve full sequence coverage during peptide mapping studies.
LC-MS-based peptide mapping offers an in-depth understanding of the peptide sequence. This analysis can confirm the identity of the primary sequence, characterize modifications to the amino acids in the sequence such as post-translational modifications (PTMs) and glycosylations, map the disulfide bonds, and elucidate misincorporations in the amino acid sequence.
Additionally, peptide mapping may be performed via LC-UV analyses. While less specific than LC-MS, LC-UV-based peptide mapping is less expensive and can be conveniently performed at a wider number of laboratories. Such analyses are usually performed after establishing LC-MS-based peptide maps during the course of drug development, and frequently utilized as an ID test for GMP release and stability studies.
At Emery Pharma, we have extensive experience with both LC-MS and LC-UV-based peptide mapping studies, conducted on high field Orbitrap mass spectrometer
Glycan Profiling
N-Glycosylation is a co-translational process that widely occurs during the expression of biologic compounds in mammalian and eukaryotic cells, but rarely in bacterial cells. A variety of different oligosaccharides (glycans) are bound to the biologic molecule, and this is called the glycan profile.
It is important to identify the glycans throughout the development cycle of the biopharmaceutical for several reasons. The glycan profile impacts the safety and efficacy of the biologic compound, and a consistent glycan profile during development and commercialization indicates process stability. Additionally, an immune response can be triggered by the appearance of an unrecognized glycan. The glycan profile also reflects the health of the cells during the protein expression process.
Emery Pharma has expertise in several methodologies that characterize the variety of glycans which are bound to the biologic. The simplest protocol involves intact and fragment mass analyses, however, only the most abundant glycans are observed by this method. The definitive and most sensitive technique involves the enzymatic release of the glycans, followed by labeling of the glycans and injection into the UPLC-MS system. The identities of the glycans are confirmed by both mass spectrometry and by comparison of retention times to authentic standards.
Host Cell Proteins (HCPs)
Host cell proteins (HCPs) are protein impurities found in the biopharmaceutical product, which are produced by the cell lines used to express the biologic drug. HCPs are characteristic of the expression system and are normally present in minor/residual amounts in the final product. HCPs in any biologic drug product need to be closely monitored as they may cause immunogenicity in individuals, reduce the potency, stability, or overall effectiveness of the biologic. HCPs are monitored throughout the development process and the method is validated prior to commercialization.
Emery Pharma characterizes the HCP impurities by utilizing modern UPLC-MS techniques and methods with a high field Orbitrap mass spectrometer during process and product development. Once the process is established, Emery Pharma has a full suite of bioanalysis tools, e.g., LC-MS/MS, ligand binding assays, ELISA, etc. to perform HCP assays.
Size Exclusion Chromatography (SEC), Ion Exchange Chromatography (IEX), and other HPLC Analysis of Biologics
Size Exclusion Chromatography (SEC) and Ion Exchange Chromatography (IEX) are specialized HPLC techniques that are workhorse methods in the analysis of biologic compounds used for release and stability testing. Emery Pharma has expertise and equipment to provide analyses and interpretations of all aspects of these methods.
SEC methods are useful for assessing size variation and aggregation, while IEX methods are useful for assessing charge variants and purity. IEX methods are also frequently used for ID testing compared to an authentic standard. These methods can also be scaled up to provide semi-prep isolation and characterization of the observed components, and to verify chromatographic purity of the main component.
Additionally, reversed-phase HPLCX methods are very useful for ID testing, and potentially for stability-indicating methods.
All HPLC analyses at Emery Pharma is conducted on HPLC and UPLC systems from Thermo Scientific, Waters, and Agilent.
Sample Enrichment
Sample enrichment is crucial in biologics investigation, enhancing analysis depth by selectively increasing the concentration of specific components. This process improves sensitivity, crucial for identifying low-abundance components in early-stage biologics development. By isolating specific targets, sample enrichment facilitates a more comprehensive understanding of complex biological systems, overcoming challenges related to sample complexity and heterogeneity. The methods of enrichment vary depending on the target; for instance, phosphorylation groups may be enriched via an iMAC column, organelles through ultracentrifugation, and activity-based enrichment achieved with chemical probes. This strategic tool not only accelerates the development of innovative biologic therapies but also provides a tailored approach to unraveling molecular landscapes based on specific enrichment needs.