N-nitrosodimethylamine (NDMA) in Ranitidine: The FDA Issues a Nationwide Recall
Background
On January 2, 2020, Emery Pharma filed a Citizen Petition (Docket No. FDA-2020-P-0042) with the FDA regarding the drug Ranitidine, which appear to produce unacceptably high levels of a cancer-causing chemical when exposed to heat. Ranitidine is a common heartburn medication and is sold under various brand names, including Zantac®.
On April 1, 2020, Emery Pharma received the official response letter from the agency (see here), which states that the FDA’s own investigation supported our conclusions regarding ranitidine stability and its propensity to form the carcinogenic chemical N-nitrosodimethylamine (NDMA). Additionally, the FDA granted most of Emery Pharma’s requests made via the Citizen Petition, including to recall, investigate, inform the public, and require manufacturers to conduct stability assessment of ranitidine drug substance and products. Please see our press release here.
Further, on April 1, 2020, FDA directed manufacturers to request removal of all ranitidine products (Zantac®) from the market. The FDA also advised consumers to stop taking any ranitidine medications, to safely dispose of them, and speak to healthcare providers to switch to safer alternatives.
How did all this get accomplished? This blog post outlines the events that led to this filing, and provides a summary of this critical experience gained by the Scientists working on the project.
Introduction to N-nitrosodimethylamine (NDMA), ranitidine, and detection of NDMA impurity in Zantac® and other ranitidine drug products by the FDA
As of September 13, 2019, the U.S. Food and Drug Administration (FDA) announced their findings of N-nitrosodimethylamine (NDMA) in a brand-name drug, Zantac®.¹ According to the International Agency for Research on Cancer (IARC), NDMA is classified as a probable human carcinogen.² NDMA is also a common environmental contaminant, which is typically examined for its presence in food and water.³ Along with NDMA, the agency has investigated other nitrosamine impurities in Angiotensin II Receptor Blockers (ARBs) since 2018.
The active pharmaceutical ingredient (API) in Zantac® is ranitidine, an H₂ histamine receptor blocker ('H2 blocker'). Ranitidine is effective in reducing stomach acid and is commonly used to treat and prevent stomach ulcers and gastroesophageal reflux. While ranitidine drug products have been on the market since the 1980’s, issues regarding the presence of NDMA have recently become evident.⁴,⁵
Emery Pharma has a long-standing interest in the study of pharmaceutical impurities. Scientists at Emery Pharma have been working on method development and validation for quantitation of NDMA, N-nitrosodiethylamine (NDEA) and several other nitrosamine impurities in APIs and drug products since early 2018. These early studies primarily focused on Valsartan®, Losartan®, and other ARBs, and utilized traditional headspace GC-MS ('HS-GC-MS') -based methodologies. Notably, Emery Pharma played an instrumental role in confirming Valisure’s data,⁵ in addition to supporting several other Clients on the topic.
Valisure, LLC is the United States’ first Analytical Pharmacy with the motto of delivering chemically batch-validated and certified medications to patients. As early as September 9, 2019, Valisure had filed an FDA citizen petition, reporting extremely high levels of N-nitrosodimethylamine (“NDMA”), irrespective of the manufacturer, manufacturing lot, dosage, and formulation of ranitidine.⁴ The FDA had established that the daily intake limit for NDMA should be less than 96 ng, whereas Valisure noted NDMA in excess of 3,000,000 ng per tablet when analyzing ranitidine products! As with the ARB testing, Emery confirmed these results prior to the submission; however, Scientists at Emery Pharma were highly skeptical about these astronomically high levels of NDMA detected. They suspected that this could be artefactual, the simple reason being that given the widespread use of ranitidine for many decades, it is highly unlikely that such high-level exposure of NDMA would remain without obvious and quantifiable health outcomes. Such obvious detrimental health effects have not been noted with ranitidine up to this date. Instead, we believed, any contamination of ranitidine had to be more subtle, and association of ranitidine use with adverse health effects need to be identified via careful epidemiological studies.
Based on additional structural analysis (vide infra), we hypothesized that the high NDMA levels may be due to inherent instability of the ranitidine molecule at high temperatures of the HS-GC-MS (130 ⁰C or higher) used for analysis. To that end, Emery Pharma started exploring alternative methods, including Liquid Chromatography ('LC') – tandem mass spectrometry ('MS/MS') and LC- high resolution mass spectrometry ('HRMS') analyses that does not require exposing the samples to high temperatures. Subsequently, FDA opined on October 2, 2019 and confirmed Emery’s theory that the HS-GC-MS methodology used in Valisure’s September 9 Citizen Petition was not suitable for ranitidine analysis, as the method requires incubation of the drug at elevated temperatures. Instead, the agency published a complementary LC-HRMS method for detection and quantification of NDMA in ranitidine drug substance and products. Using this method, FDA analysts detected much lower levels of NDMA in ranitidine, yet still found unacceptable levels of NDMA in some lots of the drug.
Emery Pharma develops and validates a novel LC-MS/MS method for quantitation of NDMA in ranitidine API and drug products
Independent of the FDA and several months prior to the agency providing any methodological details, Emery Pharma had initiated the development and validation of an LC-MS/MS method for the detection and quantification of NDMA in drug products, including Zantac®. LC-MS/MS was chosen over LC-HRMS due to its better dynamic range and specificity for NDMA detection and quantitation. The method details are provided in Tables 1, 2, and 3.
Table 1. Liquid chromatography (LC) conditions used for the analysis of NDMA in ranitidine drug substance and products.
| Ultra-High Performance Liquid Chromatography (UHPLC) | Agilent 1290 Infinity UHPLC system |
| Column | Agilent Zorbax SB-Aq, 2.1 x 100 mm, 1.8 µm particle size |
| Column Temperature | 37 °C |
| Flow Rate | 0.4 mL/min |
| Mobile Phase A | 5 mm Ammonium acetate in LC-MS-grade water |
| Mobile Phase B | LC-MS-grade acetonitrile |
Table 2. Source parameter conditions used for the analysis of NDMA in ranitidine drug substance and products.
| Source | Agilent Jet Stream Electrospray Ionization (AJS ESI) |
| Drying gas flow rate | 11 L/min |
| Drying gas temperature | 300 °C |
| Sheath gas flow rate | 11 L/min |
| Sheath gas temperature | 250 °C |
| Nozzle Voltage | 500 V |
| Nebulizer pressure | 60 psi |
| Capillary Voltage | 2500 V |
Table 3. Multiple Reaction Monitoring (MRM) transitions and MS conditions for the identification of NDMA in ranitidine drug substance and products.
| Precursor Ion
(m/z) |
Product Ion
(m/z) |
Fragmentor Voltage (V) | Collision Energy (eV) | Retention Time (min) | Delta EMV
(V) |
| 75.1 | 43.3 | 48 | 17 | 1.20 | 400 |
| 75.1 | 58.3 | 60 | 15 | 1.20 | 400 |
Accelerated stability studies and experimental proof for ranitidine instability
We initially evaluated the possibility of the presence of NDMA in ranitidine as a process impurity, i.e. getting generated during the synthesis of ranitidine as a side-product, as is the case with Valsartan and other ARBs. However, upon further investigation, we were unable to identify any such steps during ranitidine synthesis or purification that may produce NDMA as a by-product.
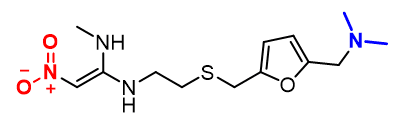
Further, the fact that HS-GC-MS showed high NDMA levels made a strong case that the ranitidine molecule may in fact be generating NDMA upon exposure to heat. As noted in Figure 1, this is quite possible, as ranitidine structure is uniquely suitable for that, providing both dimethylamine and nitro functionalities in the same molecule.
To prove this point, we initiated accelerated stability studies as follows:
- Weighed amounts of USP ranitidine were stored at room temperature (25 °C) and elevated temperature (70 °C) for up to 12 days. Zantac® (cool mint, lot# 18G542) pills were individually stored at an elevated temperature of 70 °C for up to 14 days.
- Extraction of NDMA was performed using methanol, followed by a filtration step prior to LC-MS analysis.
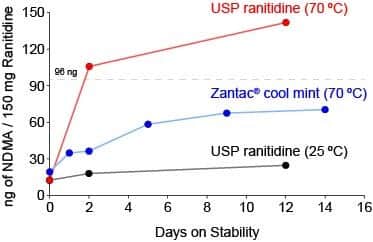
Based on the preliminary results, we found that ranitidine is indeed an inherently heat-unstable compound, and as drug substance (USP Ranitidine) or as drug product (Zantac®), the molecule undergoes degradation to form NDMA when exposed to high temperatures (Figure 2).
Figure 2. Preliminary stability study with USP ranitidine standard at 25 ⁰C (black), USP ranitidine standard at 70 ⁰C (red), and Zantac® cool mint, lot# 18G542 at 70 ⁰C (blue). NDMA analysis performed via LC-MS/MS and quantified based on a calibration curve generated using NDMA reference standard. Dotted line approximately indicates the daily intake limit of 96 ng for NDMA.
The decision to file the FDA Citizen Petition
Since FDA’s initial confirmation that certain lots of ranitidine may indeed be tainted with NDMA, most ranitidine products were on voluntary recall. However, on December 4, 2019, while Emery Pharma was still in the preliminary stages of the accelerated stability assessment, the agency offered a directive for manufacturers regarding ranitidine product release. In this directive, the agency noted that ranitidine drug products may be released back into the market, so long as production batches show NDMA levels lower than 96 ng/pill.
Needless to say, we found this directive highly concerning, for several reasons:
- At that point, to the best of our knowledge, no formal 'root-cause analysis' have been performed as to why NDMA was observed at unacceptable levels in several lots of Zantac® and other ranitidine drug products as indicated by the FDA.¹,⁶ Hence, we found an explicit directive for product release a bit premature, particularly when most manufacturers have already voluntarily recalled this drug and several alternatives already existed in the market.
- Around November, 2019, our internal (yet unpublished) results had revealed the stability concerns with ranitidine, suggesting that even if manufacturing lots were clean, NDMA may still be produced in ranitidine drugs products during downstream shipment and storage. Temperature excursions are a routine occurrence during commercial product shipment and storage of pharmaceuticals, considered among the least reliable pharmaceutical processes.⁷ And, even beyond commercial handling, drugs are routinely stored by patients under less-than-ideal conditions, e.g. keeping drugs in a car dashboard; temperatures inside a closed passenger vehicle can reach as high as 80 ⁰C during summer months! These practices are reasonably widespread, and particularly concerning for ranitidine, which carries no requirement for 'cold-chaining', i.e. shipped and stored under refrigerated conditions. We at Emery Pharma opined that if ranitidine were to be marketed, it should come with adequate warnings against improper storage for risks of generating a cancer-causing impurity.
- Finally, in this December 4 directive, the agency recommended that each ranitidine pill (usually 150 mg of active ingredient) contain no more than 96 ng of NDMA for product release. As mentioned previously that this '96 ng' is not an arbitrary number, rather this is the FDA’s recommended daily intake limit for NDMA. Whereas, depending on the stomach disease in question, ranitidine dosage may be 150 mg orally 2 times (common) or even 4 times a day, with a maximum daily dose up to 6 grams. Hence, the acceptable limit of NDMA should be at most 48 ng/150 mg ranitidine tablet (considering a 2 pill regimen), or perhaps lower! Hence, we believed that, at the very least, manufacturers should be required to ensure NDMA levels are much lower than the 96 ng/pill as indicated in the FDA’s directive.
Due to these concerns, we felt obligated to inform the agency of our internal findings. Our original plan was to complete the stability and other studies with ranitidine (scheduled to finish ~ April 2020) and submit the results to a peer-reviewed publication. But this plan had to be modified, such that some of the relevant data may be publicly shared with the FDA without jeopardizing the crux of the manuscript. It was decided, given the major public health implications of our findings, a Citizen Petition would be the ideal route to bring our data to the agency’s attention.
Hence ensued a crazy few weeks for the Scientists to get up to speed on what Citizen Petitions represent, the specific format it has to be submitted in, and get used to the fact that this is a legal, not scientific, document. Nonetheless, we powered through and managed to prepare a draft that was submitted to Emery Pharma’s legal counsel, and to our immense surprise, was approved for submission with minimal edits!
Emery Pharma’s first Citizen Petition informing the FDA on the possibility of formation of a genotoxic impurity NDMA during shipment and storage of ranitidine drug products, was submitted on January 2, 2020.⁸,⁹ The document in its entirety is available here, but if you are short of time (unlikely, since you took the time to read through this post!), a brief summary is provided in this press release.
FDA’s response letter and subsequent actions
This being Emery Pharma’s first ever Citizen Petition, we were not quite certain how this all goes down post-submission. Does the agency get back to you? Or, are petitions such as ours are more of an FYI type deal and the agency can choose how they act? Well, we soon got to know what happens after you submit a Citizen petition, first, the agency sent an acknowledgement within a week, and then what followed a couple of months later was what we had put in the 'remarkable/too-good-to-be-true' category!
After several months of hiatus, and while the country is dealing with the crisis caused by the SARS-COV-2 (the ‘novel coronavirus’), Emery Pharma received its official response to the Citizen Petition, posted here.
In this letter from Dr. Janet Woodcock, Director at the Center for Drug Evaluation and Research (CDER), the FDA has granted most of Emery Pharma’s requests made via the Citizen Petition to recall, investigate, inform the public, and require manufacturers to conduct stability assessment of ranitidine drug substance and products. The letter mentioned that via preliminary stability studies, Scientists at the FDA were able to replicate the major findings around ranitidine stability and came to the same conclusion as us!
The agency decided not to act on a few secondary requests we made in the Citizen Petition, such as: to provide instructions for disposal of ranitidine (FDA’s rationale: the agency’s web page on drug disposal adequately addresses this concern); as well as to direct a change of shipment methods, change labeling, and grant ‘prescription-only’ status for any ranitidine product (FDA’s rationale: in light of the actions taken by the agency on April 1, 2020, these requests were deemed superfluous).
Simultaneously, FDA send broad directives to manufacturers to remove all ranitidine products from the market immediately, and requested consumers to stop taking any ranitidine medication, and switch to alternatives. See the press release on the FDA’s response here.
Press coverage and ongoing work at Emery Pharma
The fact that a carcinogenic impurity may be forming in ranitidine, a drug many millions of people take every day since 1985, made for a sensational news story. Nonetheless, Scientists at Emery Pharma were unprepared for the barrage of press coverage this work garnered. Most notably, our results were featured nationally in CBS Morning News; the relevant segment is available here.
While our CEO and upper management handled the nonstop press calls, the work in the lab continued unabated. Since the initial filing, we have continued exploring ranitidine stability under various conditions, and a manuscript on the subject is being prepared. We have subsequently extended collaborations with Scientists at Valisure, Stanford University, and Memorial Sloan Kettering Cancer Institute on this project. Recently, we have expanded our investigation into several additional pharmaceutical products that may produce toxic impurities and have been previously overlooked. We remain eternally grateful to Dr. David Light (Valisure) and Prof. William Mitch (Stanford University) for sharing their expertise and providing helpful guidance as we continue on this path.
If you have any questions related to this project or our data, please feel free to reach out to the authors, Dr. Eshani Nandita and Dr. Neelanjan Bose by sending your message through Emery Pharma’s contact us page.
From Emery Pharma’s business standpoint, we are now offering resources including analytical (LC-MS/MS), as well as litigation and expert witness support to several Clients to help mitigate risk associated with the presence of NDMA in drug products such as Valsartan, Zantac, and Metformin. If you are interested in any of these services, please reach out to our Business Development Manager, Ms. Neeku Mahdavian, or our CEO, Dr. Ron Najafi, through the same contact us page.
Any press-related inquiries may be directed to Neeku Mahdavian (neeku@emerypharma.com).
About the authors
Eshani Nandita, Ph.D. is currently the Assistant Director of Bioanalytical Chemistry at Emery Pharma involved in the development of bioanalytical assays for quantification of biomolecules using LC-MS techniques. Dr. Nandita has been primarily involved in the method development and validation for NDMA quantitation and conduction the stability assessment of ranitidine.
Dr. Nandita received her Ph.D. in Chemistry (Analytical) from University of California, Davis, where she developed LC-MS methods for quantitation and structural elucidation of carbohydrates. She received her B.S. in Chemistry from California State University, Stanislaus.
Neelanjan (Neel) Bose, Ph.D. is the Director of Bioanalytical Chemistry and oversees all analytical and bioanalytical chemistry projects at Emery Pharma. Dr. Bose supervised the research activities related to ranitidine stability, LC-MS/MS-based method development/validation for NDMA and other nitrosamines in pharmaceuticals, and authored the first draft of the Citizen Petition (Docket No. FDA-2020-P-0042).
Dr. Bose received his B.Sc. (Hons.) in Chemistry from Presidency College, University of Calcutta, Kolkata, India; M.S. in Chemistry from the Indian Institute of Technology, Kanpur, India; and his Ph.D. in Chemical Biology from Cornell University, Ithaca, NY. Prior to Emery Pharma, Dr. Bose was a post-doctoral scholar at the University of California, San Francisco working on developing new analytical workflows in conjunction with invertebrate and vertebrate model systems for studying disease pathways and drug discovery in the metabolic disease space.
References:
- Woodcock, J.; Statement alerting patients and health care professionals of NDMA found in samples of ranitidine. https://www.fda.gov/news-events/press-announcements/statement-alerting-patients-and-health-care-professionals-ndma-found-samples-ranitidine.
- IARC; IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono17.pdf.
- EPA; Technical Fact Sheet – N-Nitroso-dimethylamine (NDMA). https://www.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_contaminant_ndma_january2014_final.pdf.
- Valisure; Valisure Citizen Petition on Ranitidine. https://www.valisure.com/wp-content/uploads/Valisure-Ranitidine-FDA-Citizen-Petition-v4.12.pdf.
- Edney, A. Fourth Carcinogen Discovered in Heart Pills Used by Millions. https://www.bloomberg.com/news/articles/2019-06-18/fourth-carcinogen-discovered-in-heart-pills-used-by-millions.
- FDA; Laboratory analysis of ranitidine and nizatidine products. https://www.fda.gov/drugs/drug-safety-and-availability/laboratory-tests-ranitidine.
- Ammann, C. Handling Temperature Excursions and the Role of Stability Data. https://www.pharmoutsourcing.com/Featured-Articles/146648-Handling-Temperature-Excursions-and-the-Role-of-Stability-Data/.
- Emery Pharma; Emery Pharma Citizen Petition. https://emerypharma.com/ep-ranitidine-fda-citizen-petition-v21-january-2-2020/.
- CBS; California lab finds new clues in popular heartburn drug's possible cancer link. 2020.