Importance of Poloxamer Analysis in Gene Therapy Products
If you are developing a gene-therapy-based product, which requires a Poloxamer quantitation assay, and if you are a sponsor of investigational new drug applications (INDs) or applicant of new drug applications (NDAs) that require Poloxamers testing as part of your clinical trials, or gene therapy regulatory requirement, the below communication is crucial for you.
Pluronic or Poloxamer is a synthetic amphiphilic copolymer based on hydrophilic poly-(ethylene oxide) (PEO) blocks, and hydrophobic poly-(propylene oxide) (PPO) blocks organized in a triblock structure PEO–PPO–PEO. Different types of Poloxamers, such as P-188, P-338, and P-407, are routinely used in various clinical and pharmaceutical applications. Thus, it is of vital importance to establish a bioanalytical assay with excellent analytical performance such as accuracy, precision, and specificity for the quantification of trace amounts of Poloxamer in biological matrices.
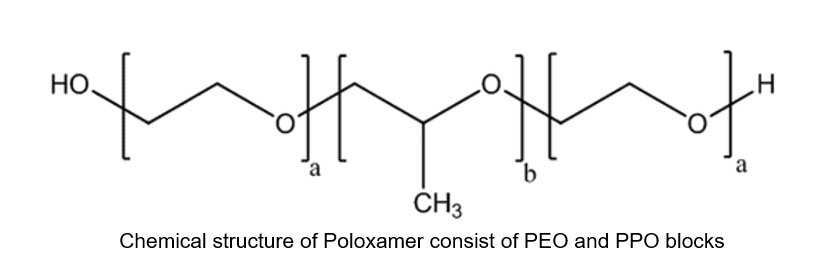
Emery Pharma has been engaged with bioanalytical testing of Poloxamers in various biological matrices for the past several years. We recently published a technical blog regarding a bioanalytical assay for Poloxamer quantitation and detection in biological matrices [1]. However, in one of the recent analyses involving a pharmacokinetics (PK) study, we encountered a great deal of interference in the pre-dose (blank) serum samples, which resulted in inaccurate quantification and invalidated consecutive analysis of the study samples.
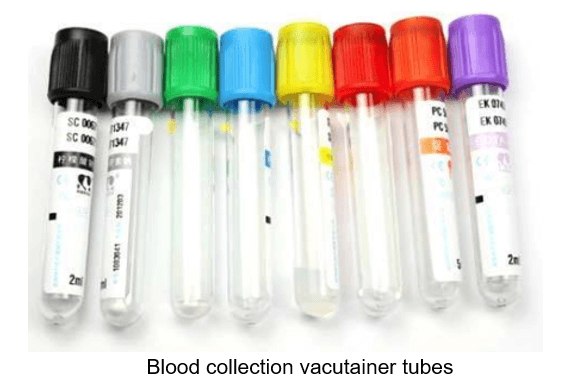
In an attempt to explore the root cause of this interference, Emery Pharma performed an extensive investigation to identify the source(s) of this Poloxamer contamination. To that end, Emery Pharma tested all possible supplies used at the clinical site, including syringes, needles, blood collection tubing and containers, gloves, etc. Surprisingly, our team narrowed down the source of contamination to some of the vacutainer blood collection tubes containing additive/surfactant used to promote clotting.
The blood collection vacutainer tubes used in the clinical site were obtained from the same manufacturer. In our investigational study, we evaluate the performance of all available versions of these tubes such as plastic and glass. According to the product specification, these tubes contain several components, including stoppers, lubricants, surfactants, and separator gels. In addition, these tubes are all commonly coated with silicone and micronized silica particles to accelerate clotting.
These tubes can potentially be a source of pre-analytical error due to leaching of unwanted materials into the study specimen. According to Bowen et al. [2], these silicone-coated materials used in these tubes are structurally similar to nonionic polydimethylsiloxane (PDMS)-polyethylene oxide and polypropylene oxide graft copolymer. The copolymer consists of a Poloxamer-like block grafted to the PDMS moiety. Hence, this copolymer might be the exact source of interference in the Poloxamer analysis.
It is imperative to validate the bioanalytical test method via evaluating the nature of interactions between collection devices and target specimens, and any potential interference this may cause during analysis. Currently, we are evaluating alternative collection devices and systems, to eradicate any possible interferences with Poloxamer detection and plan to publish a more detailed study in the near future.
At Emery Pharma, we are ready to engage our expertise and experiences in establishing a robust and sensitive bioanalytical assay for Poloxamer detection/quantification in a variety of conditions and matrices, including blood, serum, plasma, and gene therapy products. For more information, please contact us online or call us at +1 (510) 899-8814!
About the Author
Originally authored by Ali Najafi. This article was reviewed and updated on July 24, 2025 by Dr. Prajita Pandey, current Associate Director of Chemistry.
References
- Najafi, Ali. “Bioanalytical Assay for Poloxamer Detection.” Microbiology and Cell Biology, Medicinal Chemistry- Emery Pharma, 21 Mar. 2019, https://emerypharma.com/blog/bioanalytical-assay-poloxamer-detection/.
- Bowen, Raffick A.r., and Alan T. Remaley. “Interferences from Blood Collection Tube Components on Clinical Chemistry Assays.” Biochemia Medica, 2014, pp. 31–44., doi:10.11613/bm.2014.006

