Nailing the Science Behind CBD
Introduction
Cannabidiol (CBD) is a non-psychoactive compound, derived from the Cannabis plant, that can be extracted either for medicinal use or as a nutritional supplement. Over the years, there is accumulating evidence that cannabinoids are beneficial for a range of conditions, such as pain, inflammation, epilepsy, sleep disorders, multiple sclerosis, anorexia, schizophrenia, and CBD itself is generally well-tolerated with a good safety profile. In the 2018 Farm Bill, hemp—defined as cannabis and cannabis derivatives with no more than 0.3% THC (Tetrahydrocannabinol) by dry weight— was removed from the US Controlled Substance Act. In the same year, the US FDA approved the first CBD-based drug to treat two severe forms of epilepsy: Lennox-Gastaut syndrome and Dravet syndrome1.
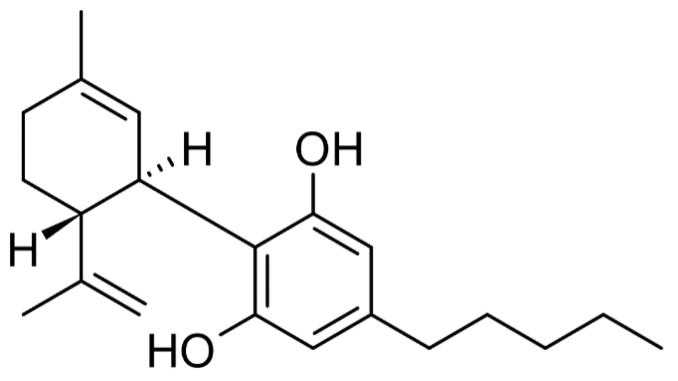
Figure 1a. Molecular structure of CBD.
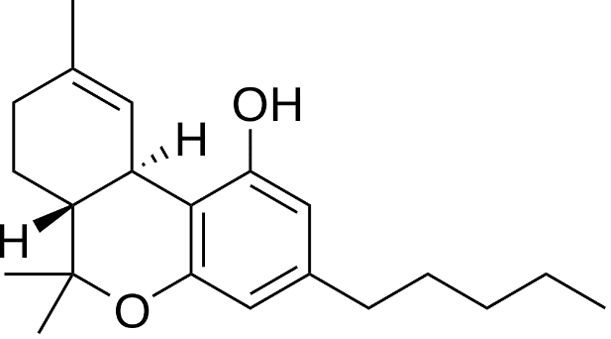
Figure 1b. Molecular structure of THC.
As a result of these legal changes, there is increasing public and clinical interest in using exogeneous cannabinoids for a variety of commercial applications, ranging from hand creams, to coffee, to dietary supplements. Many of these products purportedly alleviate anxiety, reduce inflammation, and improve sleep. However, outside of the epilepsy indication, the lack of well-controlled clinical trials, low oral bioavailability, long half-life, and highly lipophilic nature of these compounds are major impeding factors for the development of this class of compounds. There is also interest in the use of cannabinoids for diseases and symptom management, but limited information is available regarding their pharmacokinetic (PK) and pharmacodynamic (PD) properties.
Despite these challenges, further research into CBD can potentially reveal a wide range of therapeutically promising pharmacological effects, either as an individual drug or in combination with other drugs. But even with a lack of understanding in the mechanism of action2, the cannabis market continues to grow following decriminalization and legalization efforts spearheaded by state legislation. This market includes both recreational sales and novel formulations designed to take advantage of the beneficial biological effects of cannabis.
However, for drug applications, one of the challenges researchers face is differentiating between CBD and its stereoisomer, Δ-9-THC. Not only are CBD and Δ-9-THC two of the more notable cannabinoids found in cannabis with similar structures, they are both also attributed to beneficial and psychoactive effects. The use and approval of botanical drug substances for medicinal benefits—including CBD—is challenging because of the inconsistency with correlating single compound amounts to pharmacodynamic effects3. For recreational use, challenges arise with inconsistencies in label accuracy for cannabis products4, limiting the potential development of this class of compound as a drug-like molecule5.
Methods
Quantifying the amounts of CBD and Δ-9-THC thus becomes a very important factor in understanding cannabis product potential. On top of that, the short half-life of the major metabolites formed from CBD and Δ-9-THC may require additional analysis to examine the toxicity profile of these two compounds. These components are critical for determining beneficial attributes as well as the possible psycho-activity.
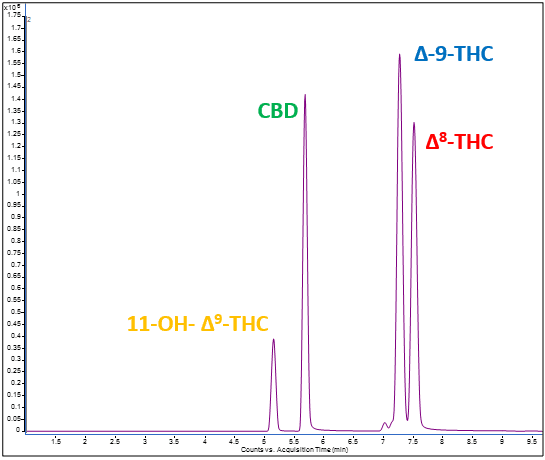
Fortunately, the laboratory here at Emery Pharma has the analytical capability of identifying and quantifying 4 major components of cannabis. One of these major components is a metabolite formed within our body called 11-OH-Δ-9-THC, a compound that can be as equally potent as Δ-9-THC6. On top of that, many new products were recently identified to contain a compound called Δ-8-THC, which also happens to have a similar biological effect as Δ-9-THC7. We have developed analytical methods for isolation and identification for these products and can achieve low ng/mL sensitivity for all of these compounds. This knowledge of chemical composition and their metabolism is a key factor for the development of botanical drugs as therapeutic agents. Additionally, Emery Pharma has the capability to assess the biological activity and pharmacokinetic (PK) properties of these compounds.
Figure 2. Representative LC-MS/MS chromatogram detecting 11-OH-Δ-9-THC, CBD, Δ-9-THC, and Δ-8-THC.
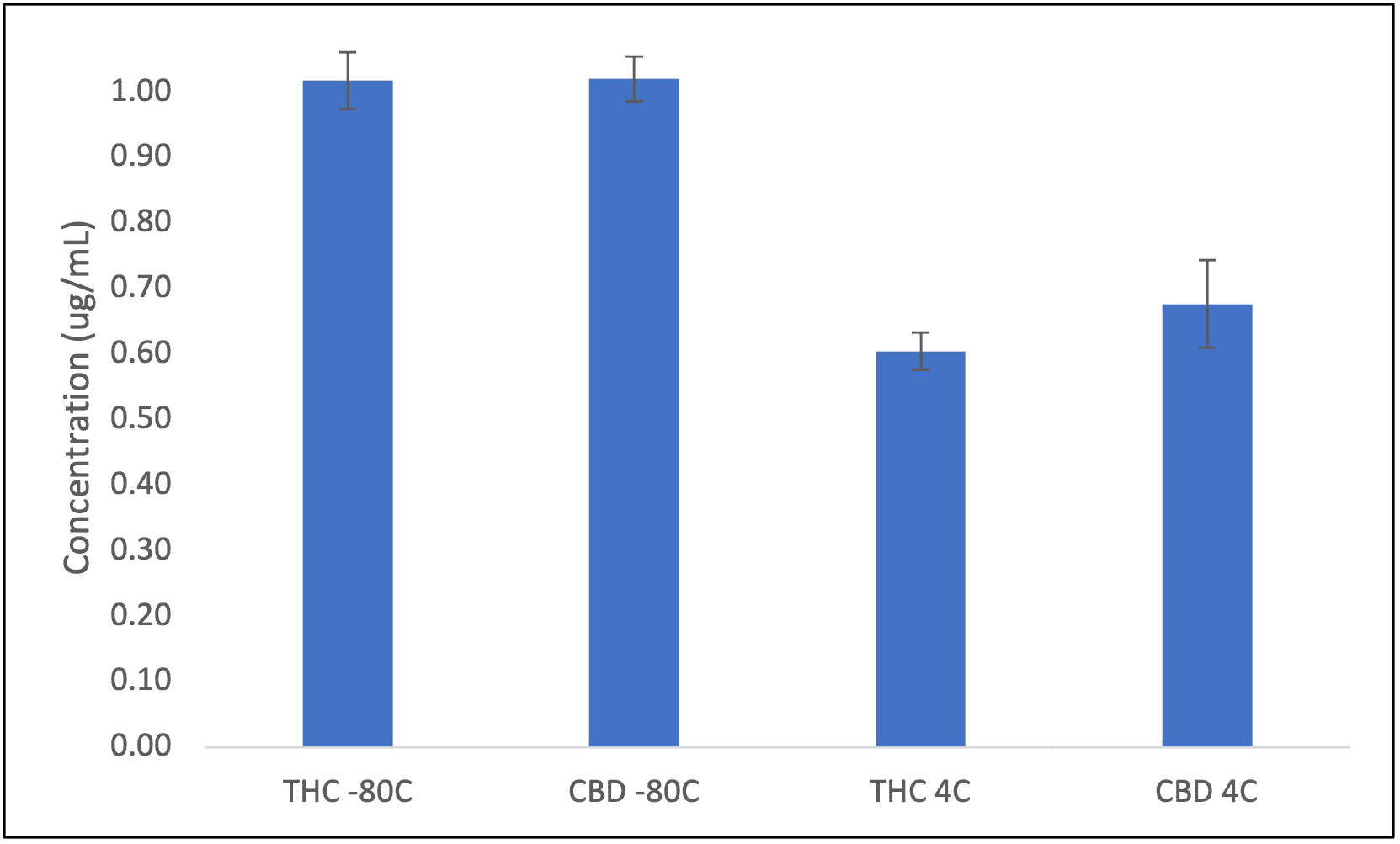
During the development of these analytical methods, Emery Pharma scientists have identified potential issues that other labs may have overlooked. Our experience has led us to investigate the critical property of stability in cannabis-derived products. Despite evidence alluding to potential temperature and light instability, many manufacturers and users often disregard these limitations8. In a recent study, Emery Pharma did a comparative side-by-side temperature stability analysis and identified significant issues in both CBD and Δ-9-THC.
Figure 3. Stability analysis of 1 µg/mL CBD and 1 µg/mL Δ-9-THC, at -80 °C and 4 °C.
In addition to its therapeutic applications, CBD and other cannabinoid compounds have been known to have antimicrobial properties against Staphylococcus aureus9, a bacteria species where there exist strains that have developed resistance to most common antibiotics, like methicillin. This finding is significant, as the spread of antibiotic resistance has been listed by as a matter of “high priority” by the World Health Organization (WHO). Even the Center for Disease Control and Prevention (CDC) has called this trend in antibiotic resistance, “…one of the world’s most urgent public health problems”10,11. With the existence of these “super bugs” and wide-spread antibiotic resistance, there is an urgent need for the identification of new antimicrobial agents that can inhibit the growth of these “super bugs” without the development of resistance. A 2019 study by Dr. Mark Blaskovich from University of Queensland, Australia found that S. aureus—as well as other Gram-positive bacteria—do not readily develop resistance against CBD12,13. This study highlights the possibility of employing CBD as a new antibiotic for the treatment of multidrug resistant Gram-positive infections.
Results
Emery Pharma has the capacity to assess antibiotic resistance and has recently completed a comprehensive antimicrobial study against S. aureus for a leading CBD oil company. Their product was a hemp-based oil extract which we evaluated to determine the Minimum Inhibitory Concentration (MIC). In antimicrobial drug discovery research, MIC screening is the first step, with MIC defined as the lowest concentration of a compound that visibly inhibits microbial growth. In this case, the inhibitory effect of the hemp oil product was tested on two strains of S. aureus: Methicillin-sensitive S. aureus (MSSA) and Methicillin-resistant S. aureus (MRSA), both of which are known to cause skin and soft tissue infections. Our results show that the MIC of the hemp oil extract against MSSA and MRSA is 2 µg/mL (Table 1), which closely matches the 0.5 to 1 µg/mL MIC value of CBD previously published in the literature9.
Table 1. MIC of hemp-based oil extract against two types of S. aureus.
| S. aureus type: | MSSA | MRSA |
| MIC of hemp-based oil extract (µg/mL) | 2 | 2 |
The next step is to then perform a time-kill kinetics assay. For this assay, the hemp extract was tested at 2-fold, 4-fold, and 8-fold above the MIC, and bacterial density was determined at 0, 6, and 24 hours. A growth control, which does not contain hemp extract, is included to monitor the natural growth of the bacteria. The results of the growth curve are shown below in Figures 4 and 5.
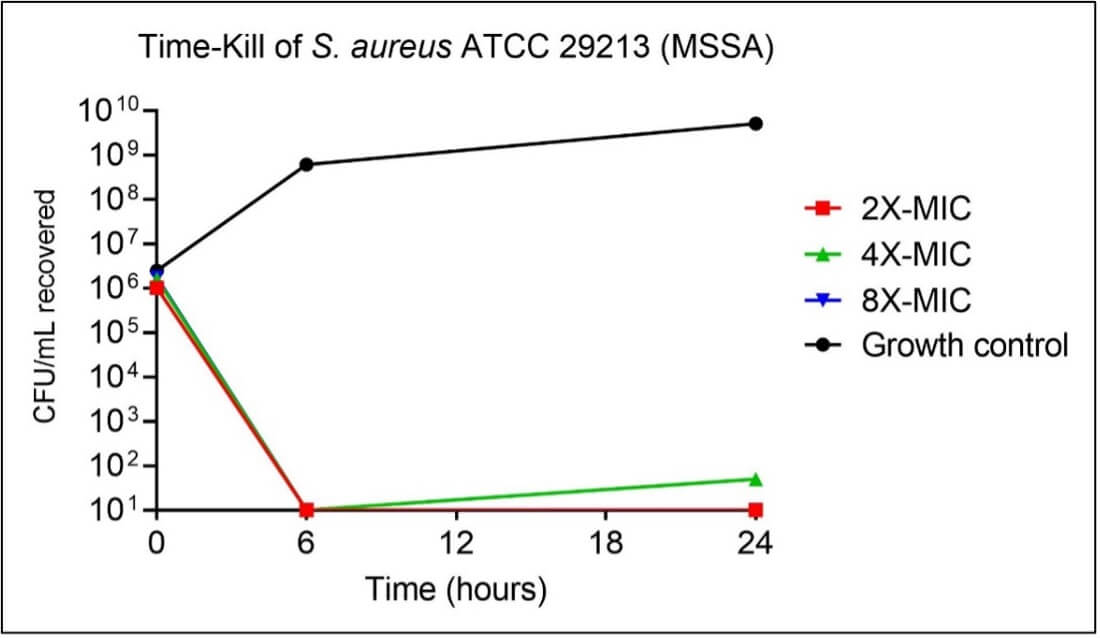
Figure 4. Time-kill of MSSA by hemp extract.
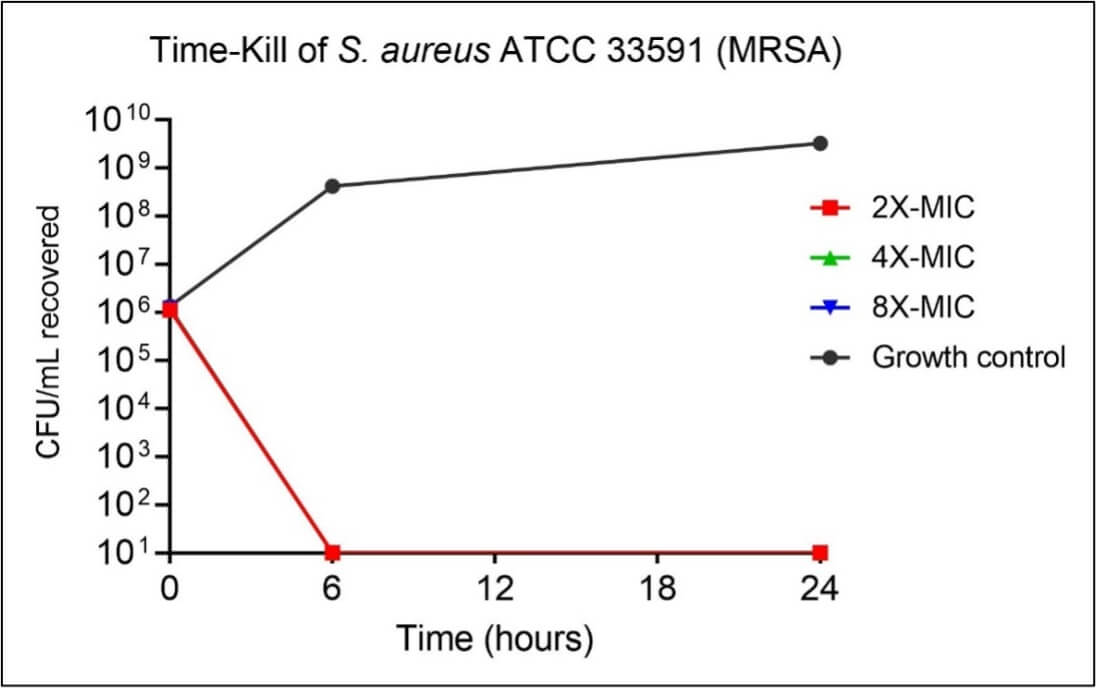
Figure 5. Time-kill of MRSA by hemp extract.
The hemp extract was able to reduce both types of S. aureus from a starting density of around 1,000,000 colony forming units (CFU) per milliliter down to less-than-or-equal-to 10 CFU per milliliter within 6 hours. This is an impressive five-thousand-fold reduction in bacterial density, and the bacteria were unable to appreciably recover and regrow by 24 hours post-contact.
Conclusion
In addition to our capabilities of assessing antimicrobial activity and chemical analysis of cannabis products, Emery Pharma is also capable of extracting and quantifying these compounds from numerous biological matrices, including saliva, plasma, urine, and stool. Additionally, we have experience designing clinical trials and conducting PK analysis from these studies, and we are also licensed to work with Schedule I to V substances, which include cannabis and Δ-9-THC.
With our experience in clinical trial design, antimicrobial activity assessment, bioanalytical evaluation of cannabis compounds, and appropriate PK analysis, Emery Pharma is ready to help you in the advancement of your drug product. If you are interested in any of these services, please contact us here to get started!
Janet Liu1 and Ryan Cheu2
1Director of Microbiology, Emery Pharma, Alameda, CA 94501, United States.
2Associate Director of Bioanalytical Chemistry, Emery Pharma, Alameda, CA 94501, United States.
References
- FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. US Food and Drug Administration (FDA). June 25, 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms
- Skelley JW, Deas CM, Curren Z, Ennis J. Use of cannabidiol in anxiety and anxiety-related disorders. Journal of the American Pharmacist Association. 2019 Dec 19. pii: S1544-3191(19)30514-X. doi: 10.1016/j.japh.2019.11.008.
- Pinto JV, Saraf G, Frysch C, Vigo D, Keramatian K, Chakrabarty T, Lam RW, Kauer-Sant'Anna M, Yatham LN. Cannabidiol as a Treatment for Mood Disorders: A Systematic Review. Canadian Journal of Psychiatry. 2019 Dec 13:706743719895195. doi: 10.1177/0706743719895195.
- Bonn-Miller, Marcel O., Mallory JE Loflin, Brian F. Thomas, Jahan P. Marcu, Travis Hyke, and Ryan Vandrey. "Labeling accuracy of cannabidiol extracts sold online." Jama318, no. 17 (2017): 1708-1709.
- CBD or THC? Common drug test can’t tell the difference. October 15, 2019. https://www.boston.com/news/health/2019/10/15/cbd-or-thc-common-drug-test-cant-tell-the-difference.
- Mura, Patrick, Pascal Kintz, Véronique Dumestre, Sébastien Raul, and Thierry Hauet. "THC can be detected in brain while absent in blood." Journal of analytical toxicology 29, no. 8 (2005): 842-843.
- Delta-8-THC: What is it and what does it do? March 29, 2019. https://growersnetwork.org/processing/delta-8-thc/
- The solubility and stability profiles of cannabidiol. June 12, 2020. https://cbdworldnews.com/2020/06/12/the-solubility-and-stability-profiles-of-cbd/#:~:text=The%20stability%20profile%20of%20CBD%20explains%20how%20the,form%E2%80%94capsules%2C%20oil%2C%20tinctures%2C%20and%20injectables%E2%80%94stability%20of%20CBD%20differs.
- Appendino G, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, Smith E, Rahman MM. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. Journal of Natural Products. 2008 Aug;71(8):1427-30. doi: 10.1021/np8002673.
- Antibiotic Resistance. World Health Organization (WHO). February 15, 2018. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
- Antibiotic/Antimicrobial Resistance (AR/AMR). Centers for Disease Control and Prevention (CDC). November 4, 2019. https://www.cdc.gov/drugresistance/about.html
- Cannabis compound could be powerful new antibiotic. University of Queensland, Australia. Institute for Molecular Bioscience. Center for Superbug Solutions. June 24, 2019. https://imb.uq.edu.au/article/2019/06/cannabis-compound-could-be-powerful-new-antibiotic
- Cannabidiol is a Powerful New Antibiotic. American Society for Microbiology (ASM). June 23, 2019. https://www.asm.org/Press-Releases/2019/June/Cannabidiol-is-a-Powerful-New-Antibiotic.