Testing Antimicrobial Activity and Cytotoxicity of Wound and Skin Cleansers
Chronic non-healing wounds, such as venous ulcers, diabetic ulcers, and pressure ulcers cause tremendous patient suffering. Treatment of such wounds presents a serious unmet medical need. Strategies that optimize the tissue repair have evolved with advances in understanding of the wound healing process (1). Infection control is an important part of these strategies. Evidence shows that a bacterial burden of 106 microorganisms or more per gram of tissue seriously impairs healing. Bacteria may stimulate a persisting inflammation leading to the production of inflammatory mediators and proteolytic enzymes. Amongst many other effects this causes Extracellular matrix (ECM) degradation and inhibition of re-epithelialisation. Recently, there had been increased interest in the role of biofilms in impaired healing. Epithelial advancement, another critical component of wound healing, requires activity of fibroblasts and keratinocytes which may be hampered by aggressive and toxic wound cleansers. When such products are used, not only bacteria are killed, but also the epithelial cells are killed. An ideal wound cleanser provides periodic reduction of bacterial contamination and removal of debris without adversely impacting cellular activities vital to the wound healing process. Therefore important steps in a comprehensive strategy for evaluating wound care products are studying their antimicrobial activity and potential cytotoxicity for relevant cell types.
Fig. 1. Bacterial biofilm results in delayed wound healing
Bacterial biofilm plays a significant role in delaying wound healing (Fig. 1)
Emery Pharma offers several models for cytotoxicity evaluation and utilizes a panel of microbial strains to study antimicrobial activity. In the example shown in Table 1, we determined the non-cytotoxic, safe concentration of several important and widely used skin/wound cleansers using cytotoxicity assay with L929 mouse fibroblasts and compared the microbicidal activity of these cleansers at their non-cytotoxic concentration against Methicillin-Resistant Staphylococcus aureus (MRSA) ATCC 33591. Cytotoxicity was evaluated by modified methods as described by Wilson et al. (2). Cells were cultured in 96-well plates to approximately 70% confluency prior to exposing them to various cleansers. Cleansers were aspirated off the wells after exposure for 30 min at 37°C. Cells were incubated in fresh media overnight prior to determining cell viability using CellTiter 96 cell proliferation assay (Fig. 2). Cleansers were serially diluted 1:10 with PBS.

Fig. 2. Testing cytotoxicity by CellTiter 96 cell proliferation assay
|
Agents Tested |
Nontoxic dilution |
Time to 4 Log10 Kill |
|
Formulation A |
100 |
>24 hrs |
|
Formulation B |
10-1 |
30 min |
|
Formulation C |
10-1 |
>24 hrs |
|
Formulation D |
10-1 |
<1 min |
Table 1. Toxicity index and antibacterial time kill kinetics of cleansers. Each cleanser was serially diluted 1:10 with PBS and each dilution was tested for cytotoxicity until the results of the cells exposed to the diluted test solutions were similar to those cells exposed to PBS alone.
Formulation A was found to be the least toxic to fibroblasts, requiring no dilution to maintain viable cells. Several formulations (B, C, D) required a 10-fold dilution.
Time-kill assay is a measure of how long it takes for an antimicrobial product to achieve an effective 4 log kill of a defined inoculum. The assay was conducted using modified CLSI methods. A standard inoculum of MRSA ATCC 33591 was grown, centrifuged, and re-suspended to approximately 108 CFU/mL before incubation with cleansers at their non-cytotoxic dilution for 1, 5, 15, 30, 60 min, 4 hr, and 24 hr. 10-fold serial dilutions of the cleansers containing the inoculum were plated on TSA, incubated overnight at 37°C and CFUs were counted (Fig. 3). Examples of these studies are shown in Fig. 4.

Fig. 3. Bacteria plated for CFU counts
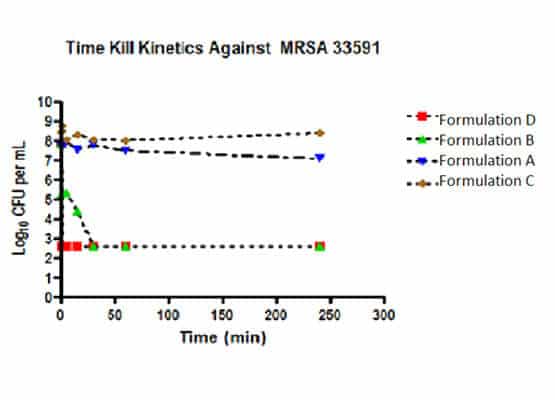
Fig. 4. The time to kill at the non-toxic dilution of Formulation D (10-fold dilution) was less than 1 min, followed by Formulation B (10-fold dilution) at 30 minutes. Formulations A and C showed no log reduction at 24 hours.
These studies are useful to clinicians developing wound care strategies and to those wishing to develop and expand in vitro methods to evaluate the potential cytotoxic and bactericidal effects of agents used for wound care. This study demonstrates that many wound and skin cleansers (Fig. 5) may be toxic to fibroblasts, one of the key cells in wound repair, and suggests that these cleansers might also be toxic to other cells. When diluted to “cell safe” concentrations, most of the cleansers lost antibacterial activity, as reflected by the length of time needed to reduce the growth of S. aureus, while some products stood out for their safety and rapid antibacterial activity.

Fig. 5. Wound and skin cleanser products
Emery Pharma also offers a wide range of additional microbiology services including resistance profiling services, antimicrobial susceptibility testing, antibiotic sensitivity, and a variety of biofilm models all of which can be adapted to suit any testing needs.
References:
1. Grotendorst GR, Pencev D, Martin GR, Sodek J. Molecular mediators of tissue repair. In: Hunt TK, Heppenstall RB, Pines E, Rovee D, eds. Soft and Hard Tissue Repair. Biological and Clinical Aspects (Surgical Sciences Series). Vol 2. New York: Praeger; 1984.
2. Wilson JR, Mills JG, Prather ID, Dimitrijevich SD. A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes. Advances In Skin & Wound Care 2005; 18:373 – 378
